Todays Catalyst 1 What is an intensive property



















- Slides: 26

Today’s Catalyst 1. What is an intensive property of matter? List two examples of intensive properties. Do not depend upon the amount of matter present; color, density 2. What is an extensive property of matter? List two examples of extensive properties. Do depend upon the amount of matter present; mass, volume

Today’s Catalyst 3. Compare these two elephants. What would be an intensive property of the elephants? What would be an extensive property of the elephants?

�https: //www. youtube. com/watch? v=88 MBCyia. PSM

Pure Substances and Mixtures

By the end of the class period today I will be able to… �Identify a piece of matter as an element, compound, homogeneous, or heterogeneous mixture based upon its properties

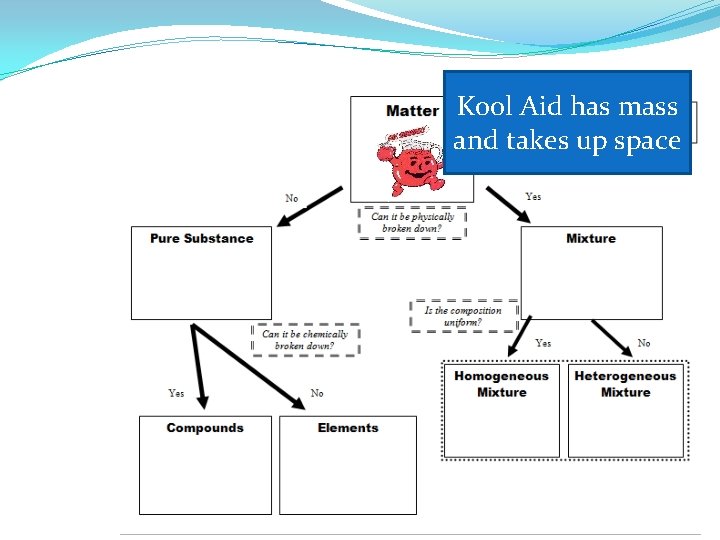
Pure Substances �Composition is the same throughout and does not vary from sample to sample. �CANNOT be broken down by physical changes �Can be an element or compound.

Element �Definition: substances in their simplest forms �Cannot be broken down by a physical or chemical change �Found on the periodic table �Made up of one type of atom

Examples of Elements: �Hydrogen �Carbon �Lithium �Gold What are two other examples of elements not listed above? What do all elements have in common?

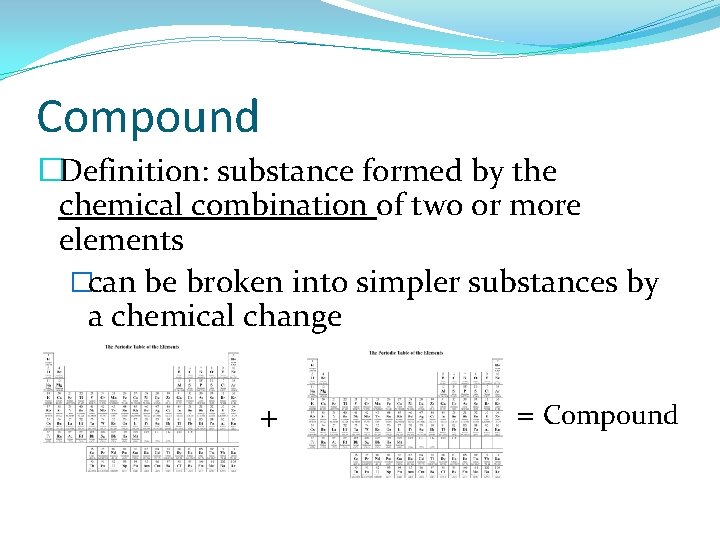
Compound �Definition: substance formed by the chemical combination of two or more elements �can be broken into simpler substances by a chemical change + = Compound

Law of Definite Proportions �A compound is always composed of the same elements in the same proportions. In other words, Carbon Dioxide (CO 2) is always composed of 1 atom of C and 2 atoms of O. If there are different amounts of carbon or oxygen, it is no longer carbon dioxide.

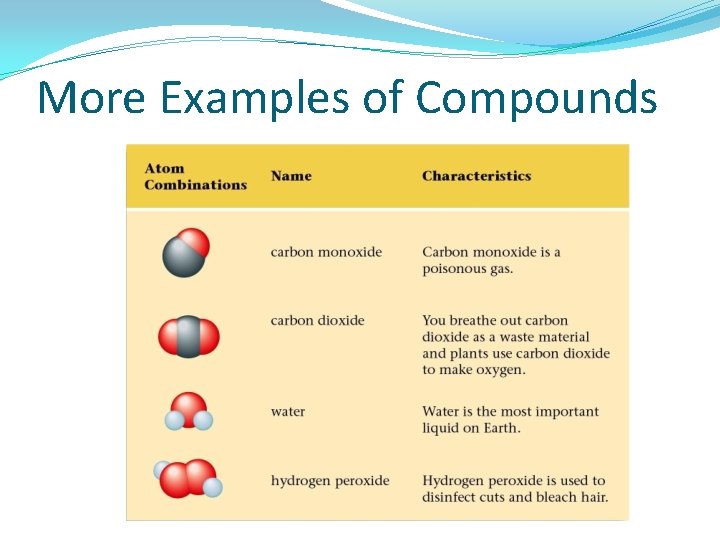
More Examples of Compounds

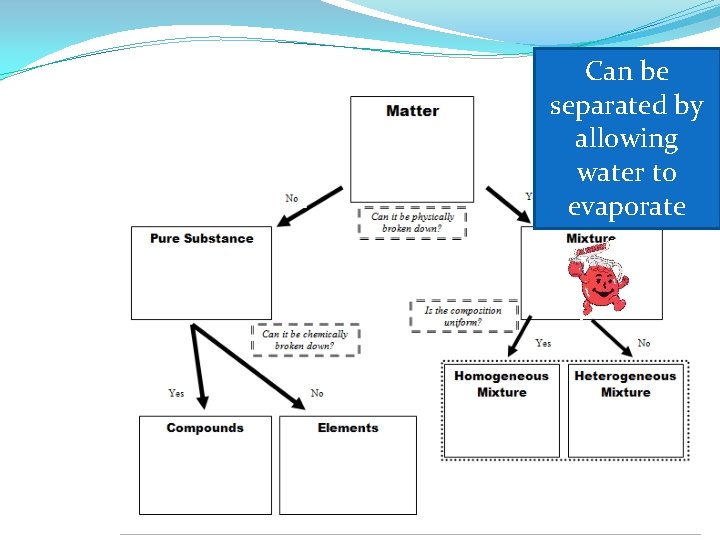
Mixture �Definition: two or more pure substances (elements or compounds) that are mixed together but NOT joined chemically �NOT a pure substance �Examples: The air we breath, gasoline for cars, the sidewalk on which we walk

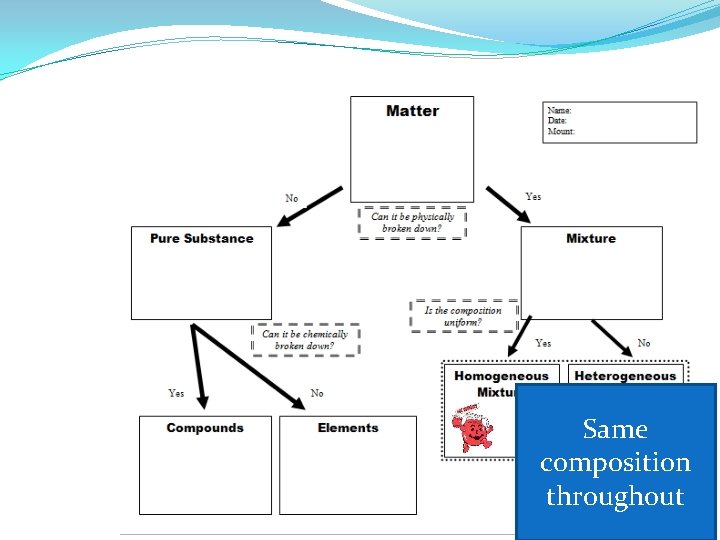
Homogeneous Mixtures �Uniform in composition and appearance �Same proportion of components throughout �Consists of two or more substances in the same phase �Also called solutions

Heterogeneous Mixtures �variable appearance and composition

Raise ‘em Up! �Look at the following example and with your partner determine if it is a heterogeneous mixture, homogeneous mixture, element, or compound

Chicken Noodle Soup Heterogeneous Mixture

Pure Water Compound

Tap Water Homogeneous Mixture

Pure Gold Element

Coca-Cola Homogeneous Mixture


Kool Aid has mass and takes up space

Can be separated by allowing water to evaporate

Same composition throughout

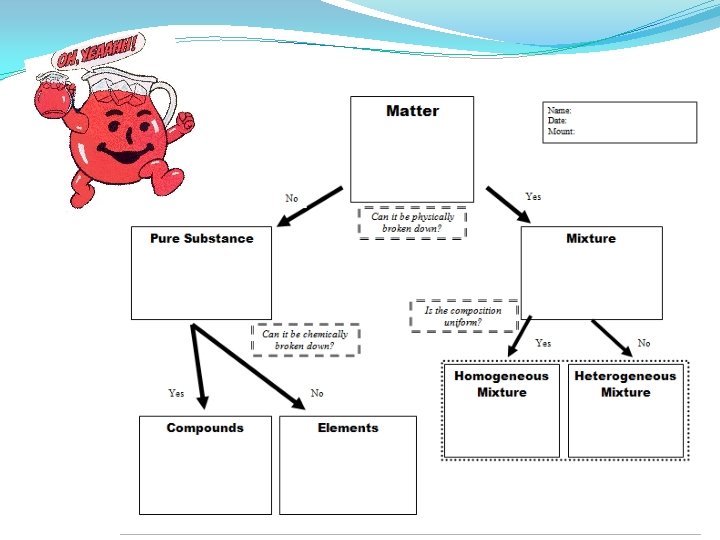
Wrapping up! �In 1 -2 STRONG sentences…under your flow-chart… �Why is it DIFFICULT to classify matter? ?

HOMEWORK!!! �Complete the 2 -sided Pure Substance and Mixtures Homework sheet – due tomorrow! We will go over ALL correct answers tomorrow!! �TEST coming THURSDAY SEPTEMBER 17 th !!! �Covers: Physical/Chemical Properties AND Changes, States of Matter, and Pure Substances and Mixtures!!