Today Wrap up Exp 1 Melting Points Introduction






- Slides: 15

Today: • Wrap up Exp. 1: “Melting Points” • Introduction to Exp. 2: “Recrystallization” (2 Lab periods) • In Lab: Today: 2 ab. Next week: 2 c and completion of 2 b Next Friday: Mini. Quiz on melting points and recrystallization (review lab, lab lectures. Do Practice Questions)

Your mp. results? Mp’s of pure substances, of mixtures? Problems? What factors affect the melting points of organic compounds?

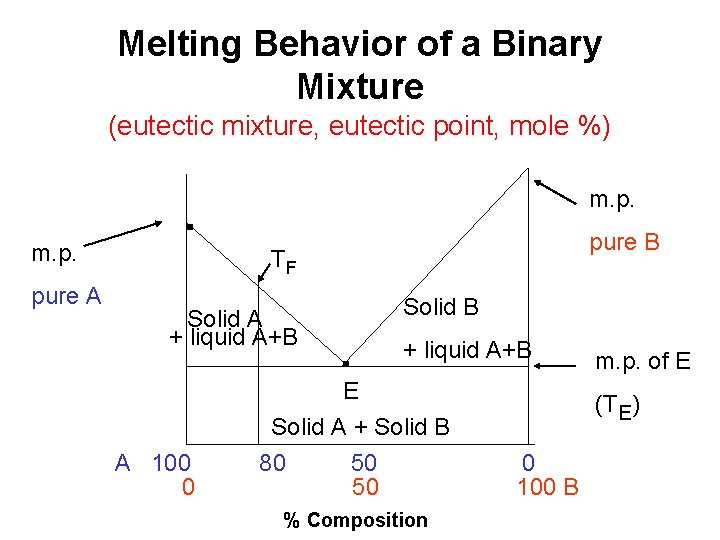
Melting Behavior of a Binary Mixture (eutectic mixture, eutectic point, mole %) m. p. pure A . m. p. pure B TF Solid A + liquid A+B A 100 0 . Solid B + liquid A+B E Solid A + Solid B 80 50 50 % Composition m. p. of E (TE) 0 100 B

Exp. 2: (Re)Crystallization: a very common purification methods of solids to isolate a pure compound from a mixture • relatively inexpensive • microscale to macroscale

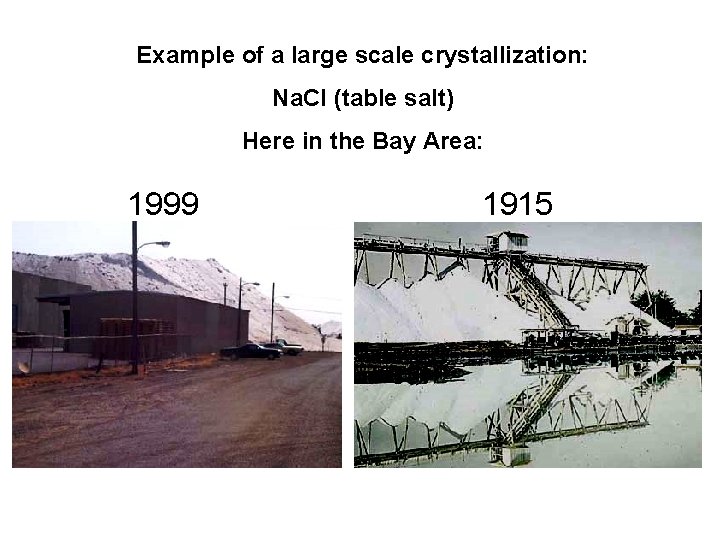
Example of a large scale crystallization: Na. Cl (table salt) Here in the Bay Area: 1999 1915

What factors affect the solubility of a solid in a liquid solvent?

Principles of Recrystallization Select a solvent that will dissolve the desired solid in the hot but not the cold solvent. Cooling should lead to crystallization again. Impurities should be well soluble in cold solvent thus stay in solution Or Impurities should be insoluble in hot solvent (filter them off while solution is hot)

General Recrystallization Procedure (see also your text) 1. Select a suitable solvent (Part 2 A) 2. (Part 2 B) Dissolve the impure solid in minimum amount of warm solvent 3. When the impure solid has completely dissolved, filter the heated solution. Evaporate a portion of the solvent to bring it to the point of saturation. 4. Cool the saturated solution to reduce solubility. Crystallization sets in. 5. Isolate the solid by filtration, then dry the crystals. How can we check the purity of the recrystallized solid?

Melting ? Dissolving

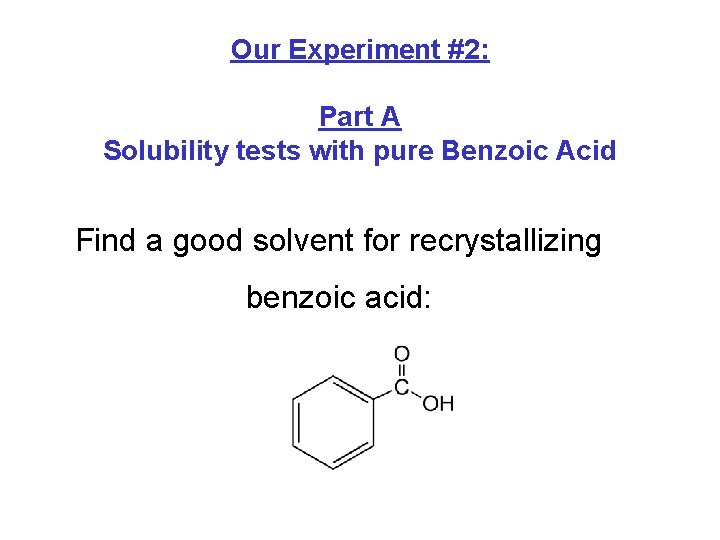
Our Experiment #2: Part A Solubility tests with pure Benzoic Acid Find a good solvent for recrystallizing benzoic acid:

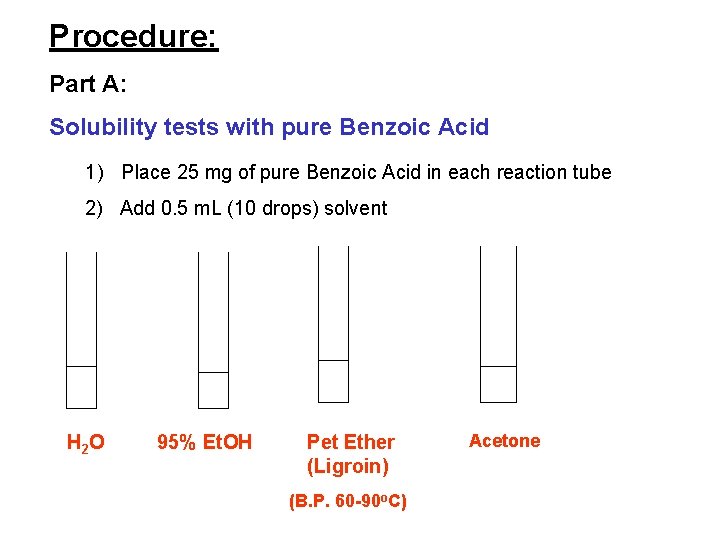
Procedure: Part A: Solubility tests with pure Benzoic Acid 1) Place 25 mg of pure Benzoic Acid in each reaction tube 2) Add 0. 5 m. L (10 drops) solvent H 2 O 95% Et. OH Pet Ether (Ligroin) (B. P. 60 -90 o. C) Acetone

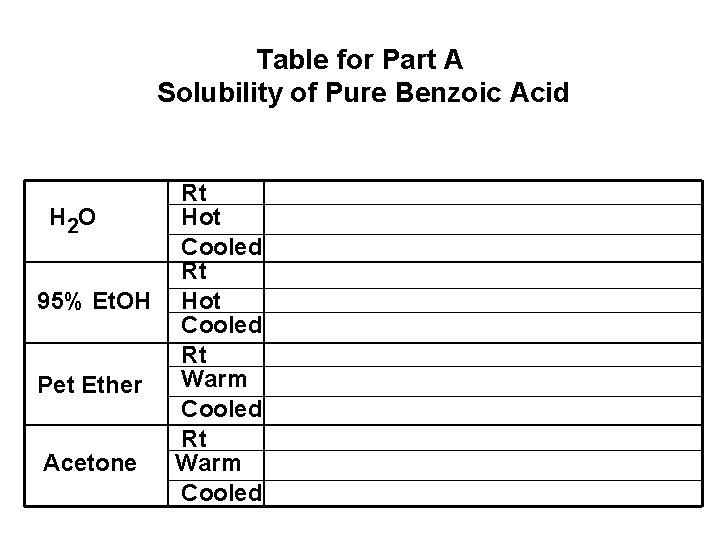
Table for Part A Solubility of Pure Benzoic Acid Rt H 2 O Hot Cooled Rt 95% Et. OH Hot Cooled Rt Warm Pet Ether Cooled Rt Acetone Warm Cooled

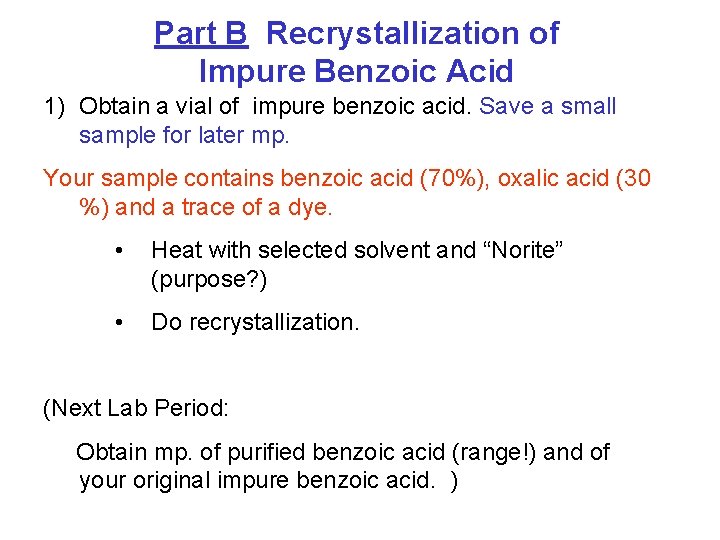
Part B Recrystallization of Impure Benzoic Acid 1) Obtain a vial of impure benzoic acid. Save a small sample for later mp. Your sample contains benzoic acid (70%), oxalic acid (30 %) and a trace of a dye. • Heat with selected solvent and “Norite” (purpose? ) • Do recrystallization. (Next Lab Period: Obtain mp. of purified benzoic acid (range!) and of your original impure benzoic acid. )

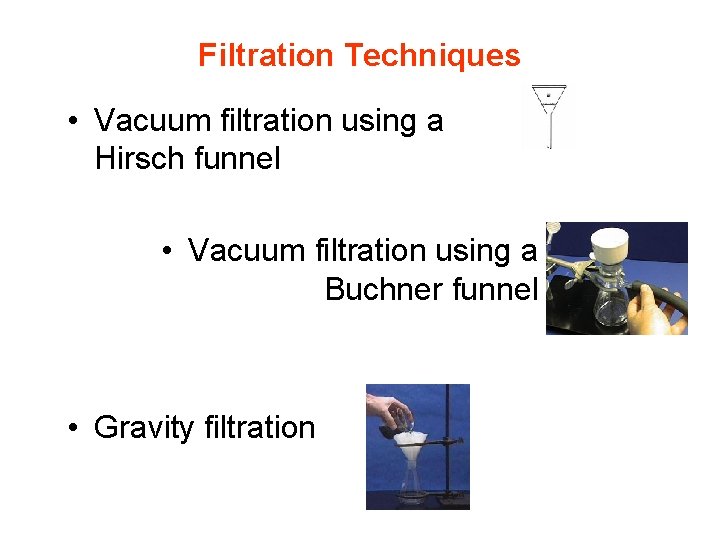
Filtration Techniques • Vacuum filtration using a Hirsch funnel • Vacuum filtration using a Buchner funnel • Gravity filtration

Next time: • Your “experiences” with recrystallization • Some calculations and practice examples for recrystallization Mini. Quiz on melting points and recrystallization! (review lab, lab lectures. Do Practice Questions)