Today Chapters 14 and 15 Temperature and thermal











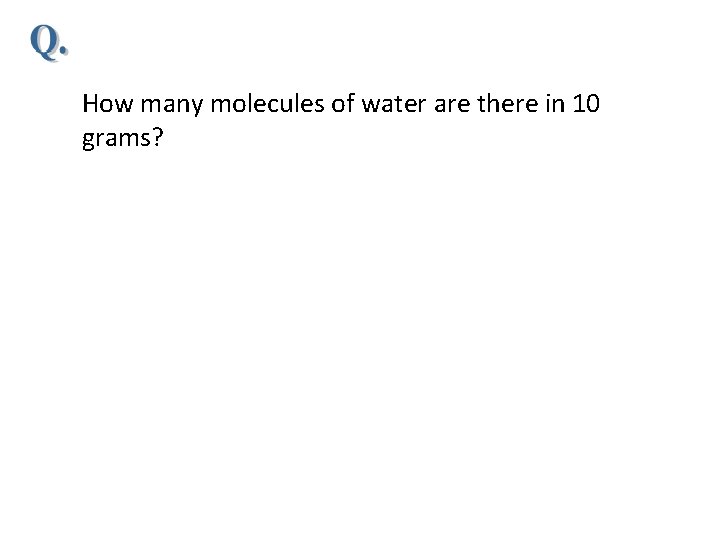
![Ideal gas law (Macroscopic form) P [Pa] V [m 3] n [mol] T [K] Ideal gas law (Macroscopic form) P [Pa] V [m 3] n [mol] T [K]](https://slidetodoc.com/presentation_image/1044a3fedd764ed1a96d6f825098d849/image-31.jpg)


- Slides: 36

Today (Chapters 14 and 15) èTemperature and thermal expansion èSpecific Heat Capacity èPhase changes and Heat èMolecular picture of a gas èIdeal gas law èKinetic theory of the ideal gas Tomorrow (Chapters 15 and 16) èHeat èInternal Energy, Kinetic Energy of Ideal Gas

Temperature Heat: The energy that flows between two objects or systems due to temperature difference between them is called heat. Thermal contact: If heat can flow between two objects or systems, the objects or systems are said to be in thermal contact. Zeroth law of thermodynamics: If two objects are each in thermal equilibrium with a third object, then the two are in thermal equilibrium with each other. Continued………. .

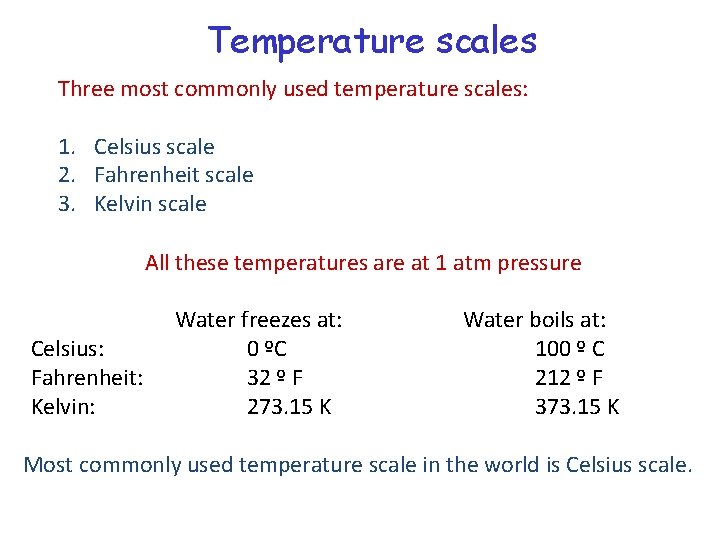
Temperature scales Three most commonly used temperature scales: 1. Celsius scale 2. Fahrenheit scale 3. Kelvin scale All these temperatures are at 1 atm pressure Celsius: Fahrenheit: Kelvin: Water freezes at: 0 ºC 32 º F 273. 15 K Water boils at: 100 º C 212 º F 373. 15 K Most commonly used temperature scale in the world is Celsius scale.

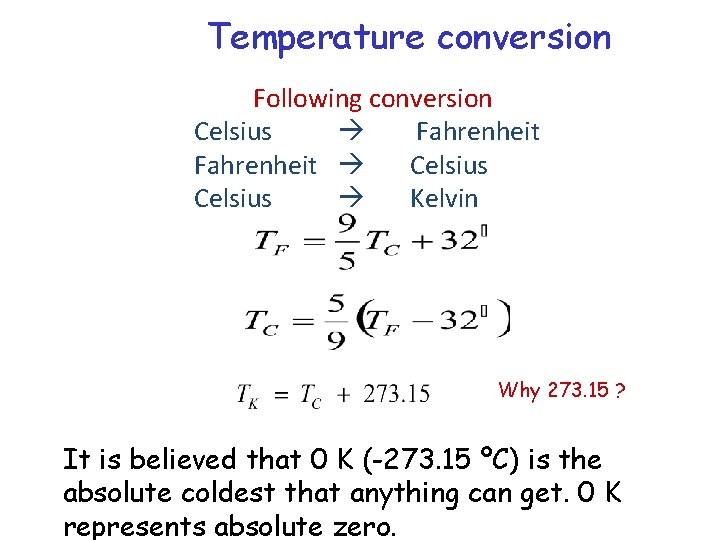
Temperature conversion Following conversion Celsius Fahrenheit Celsius Kelvin Why 273. 15 ? It is believed that 0 K (-273. 15 ºC) is the absolute coldest that anything can get. 0 K represents absolute zero.

The temperature at which liquid nitrogen boils (at atmospheric pressure) is 71 K. Express this temperature in: (a)°C (b)°F

How would you dress if the temperature outside was: (a) 70 degrees Fahrenheit? (b) 70 degrees Celsius? (c) 70 degrees Kelvin?

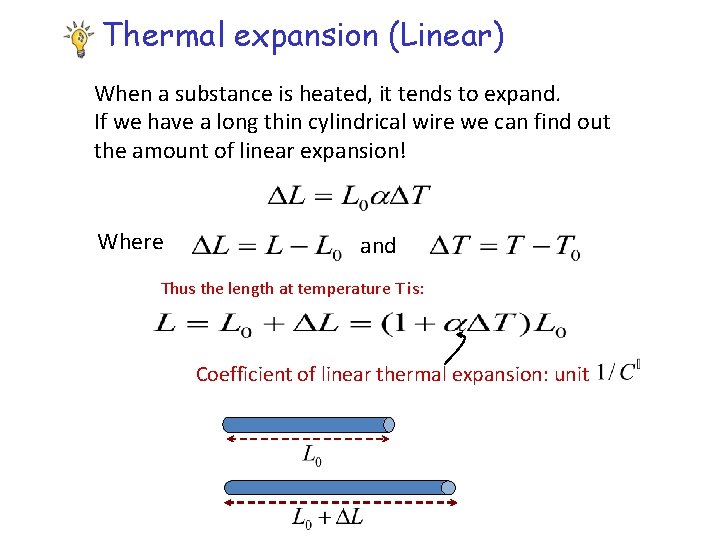
Thermal expansion (Linear) When a substance is heated, it tends to expand. If we have a long thin cylindrical wire we can find out the amount of linear expansion! Where and Thus the length at temperature T is: Coefficient of linear thermal expansion: unit

A concrete roadbed is divided into sections that are 2 meters long. How much will each section expand when the temperature changes from 0 ºC to 37 ºC (100 ºF)? Linear thermal expansion coefficient for concrete is 12 x 10 -6 /o. C. A bridge is 400 m (1/4 mile) long. How much will the steel support structure expand under the same temperature change? Linear thermal expansion coefficient for steel is 12 x 10 -6 /o. C.

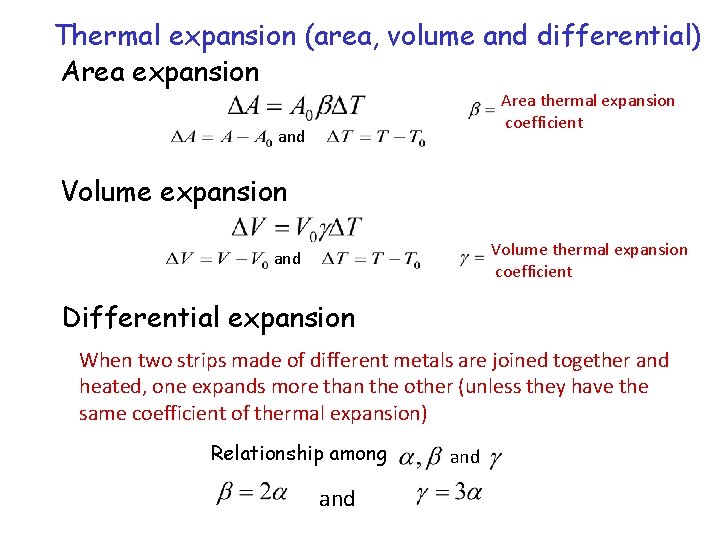
Thermal expansion (area, volume and differential) Area expansion Area thermal expansion coefficient and Volume expansion Volume thermal expansion coefficient and Differential expansion When two strips made of different metals are joined together and heated, one expands more than the other (unless they have the same coefficient of thermal expansion) Relationship among and

The coefficient of linear expansion of brass is 1. 9 × 10 -5 °C-1. At 20 °C, a hole in a sheet of brass has an area of 1. 00 mm 2. How much larger is the area of the hole at 34°C?

You place 1 cup of water (0. 000237 m 3) in a Pyrex measuring cup and place it in the microwave on high for 3 minutes. As the temperature of the water (and cup) changes from 21 ºC to 100 ºC, how does the measured amount of water change?

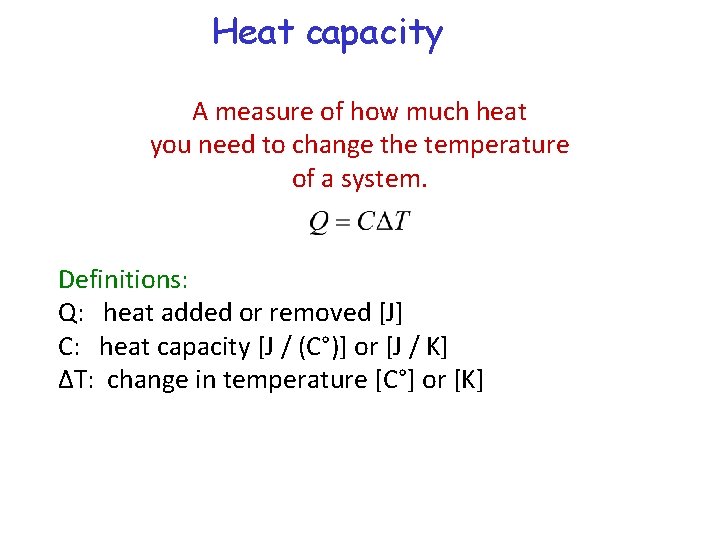
Heat capacity A measure of how much heat you need to change the temperature of a system. Definitions: Q: heat added or removed [J] C: heat capacity [J / (C°)] or [J / K] ∆T: change in temperature [C°] or [K]

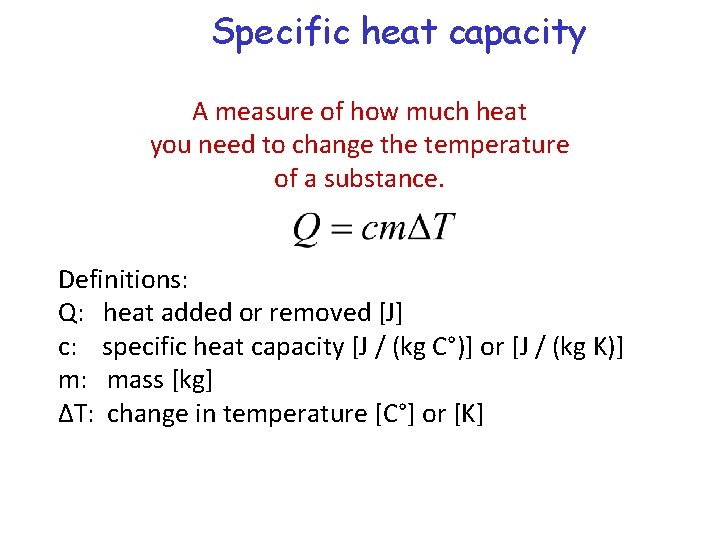
Specific heat capacity A measure of how much heat you need to change the temperature of a substance. Definitions: Q: heat added or removed [J] c: specific heat capacity [J / (kg C°)] or [J / (kg K)] m: mass [kg] ∆T: change in temperature [C°] or [K]

calories or Calories? calorie: (cal) the amount of heat needed to raise the temperature of 1 gram of water by 1 C°. kilocalorie: (kcal) the amount of heat needed to raise the temperature of 1 kilogram of water by 1 C°. Calorie: (Cal) the same as 1 kcal. Used as a measure of the amount of energy in foods. 1 kcal = 4180 Joules

The specific heat capacity for a human body is about 3500 J/kg C°. A half-hour run will burn about 800, 000 J of heat. (a) If the runner does not sweat, how much will his temperature increase? (The runner has a mass of about 60 kg. ) (b) How many Calories does the runner burn?

How much heat is required to raise the temperature of a 3 kg block of steel by 50 K? On page 441 of our book, there is a table that tells us the specific heat of steel is 450 J/(kg*K).

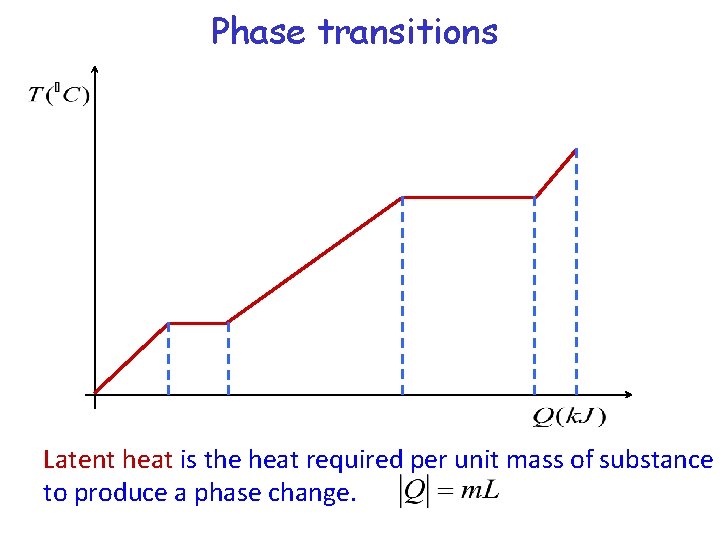
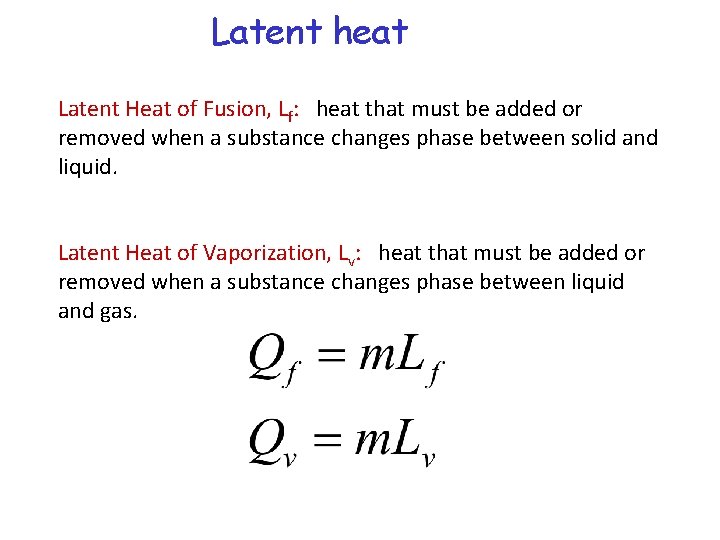
Phase transitions Latent heat is the heat required per unit mass of substance to produce a phase change.

Latent heat Latent Heat of Fusion, Lf: heat that must be added or removed when a substance changes phase between solid and liquid. Latent Heat of Vaporization, Lv: heat that must be added or removed when a substance changes phase between liquid and gas.

5, 000 J of heat are added to a 10 kg of water. If the water’s initial temperature is -4 °C, what will the water’s final temperature be?

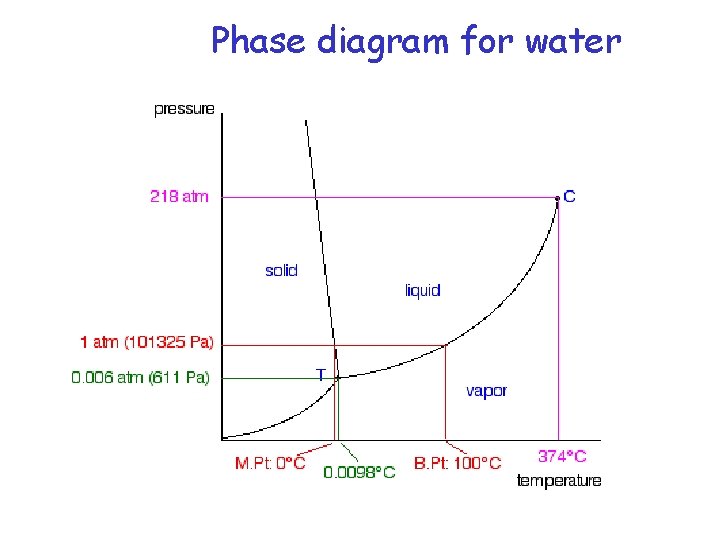
Phase diagram for water

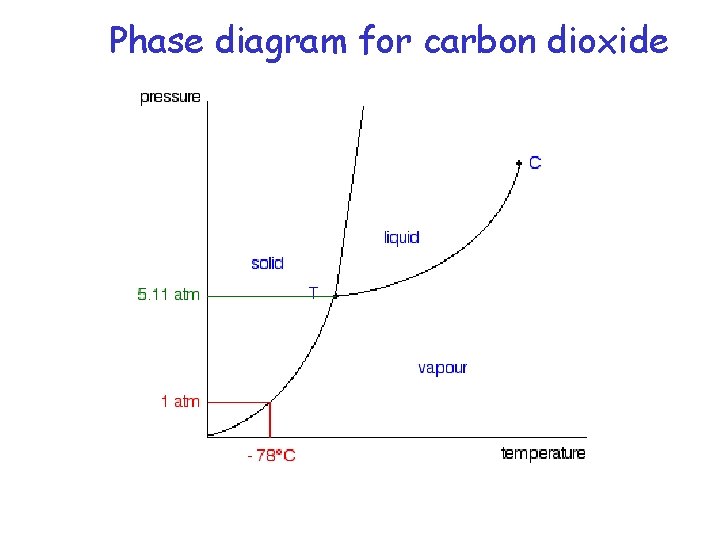
Phase diagram for carbon dioxide

A quantity of pure substance is enclosed in a container and its temperature is monitored. Describe the conditions under which each of the following behaviors can occur as heat is added to the substance. (a) As heat is added the temperature does not change. (b) The temperature rises in proportion to the amount of heat added. (c) As heat is added, there is no temperature change at first, but as more heat is added the temperature begins to rise.

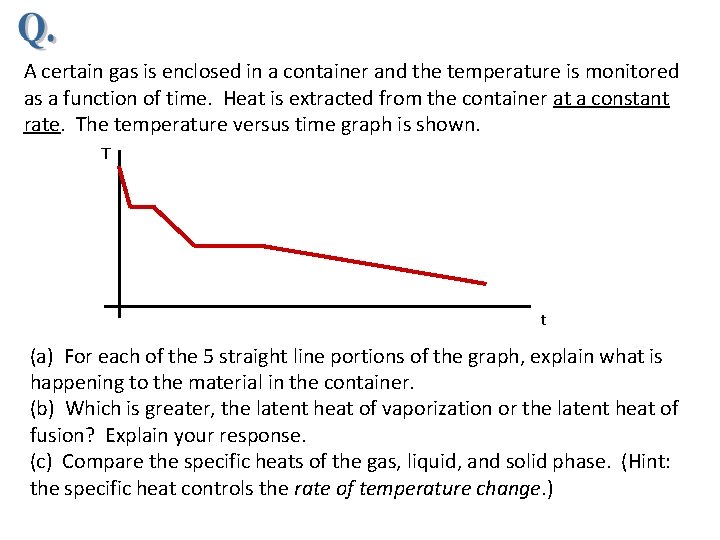
A certain gas is enclosed in a container and the temperature is monitored as a function of time. Heat is extracted from the container at a constant rate. The temperature versus time graph is shown. T t (a) For each of the 5 straight line portions of the graph, explain what is happening to the material in the container. (b) Which is greater, the latent heat of vaporization or the latent heat of fusion? Explain your response. (c) Compare the specific heats of the gas, liquid, and solid phase. (Hint: the specific heat controls the rate of temperature change. )

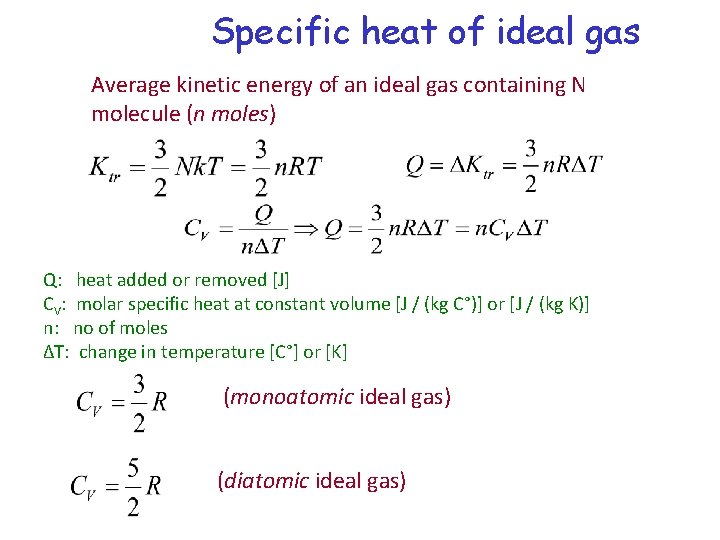
Specific heat of ideal gas Average kinetic energy of an ideal gas containing N molecule (n moles) Q: heat added or removed [J] CV: molar specific heat at constant volume [J / (kg C°)] or [J / (kg K)] n: no of moles ∆T: change in temperature [C°] or [K] (monoatomic ideal gas) (diatomic ideal gas)

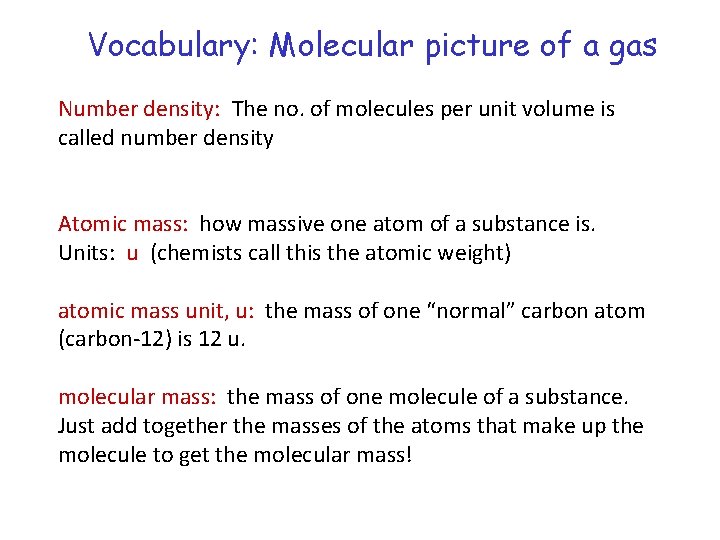
Vocabulary: Molecular picture of a gas Number density: The no. of molecules per unit volume is called number density Atomic mass: how massive one atom of a substance is. Units: u (chemists call this the atomic weight) atomic mass unit, u: the mass of one “normal” carbon atom (carbon-12) is 12 u. molecular mass: the mass of one molecule of a substance. Just add together the masses of the atoms that make up the molecule to get the molecular mass!

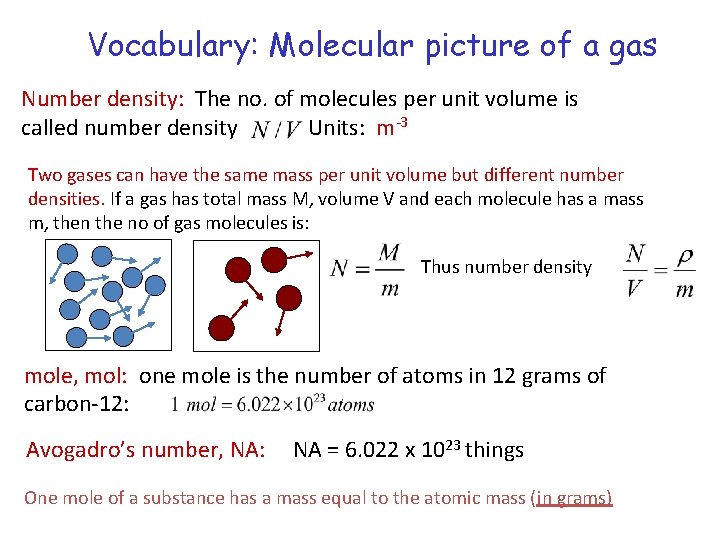
Vocabulary: Molecular picture of a gas Number density: The no. of molecules per unit volume is called number density Units: m-3 Two gases can have the same mass per unit volume but different number densities. If a gas has total mass M, volume V and each molecule has a mass m, then the no of gas molecules is: Thus number density mole, mol: one mole is the number of atoms in 12 grams of carbon-12: Avogadro’s number, NA: NA = 6. 022 x 1023 things One mole of a substance has a mass equal to the atomic mass (in grams)

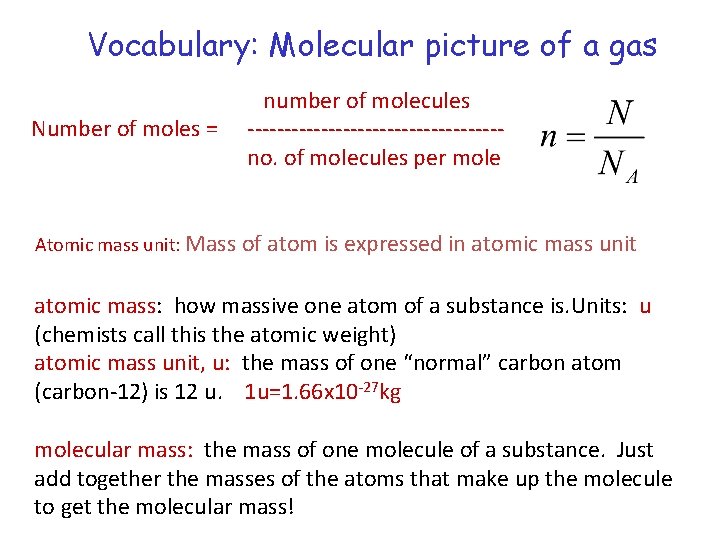
Vocabulary: Molecular picture of a gas Number of moles = number of molecules -----------------no. of molecules per mole Atomic mass unit: Mass of atom is expressed in atomic mass unit atomic mass: how massive one atom of a substance is. Units: u (chemists call this the atomic weight) atomic mass unit, u: the mass of one “normal” carbon atom (carbon-12) is 12 u. 1 u=1. 66 x 10 -27 kg molecular mass: the mass of one molecule of a substance. Just add together the masses of the atoms that make up the molecule to get the molecular mass!
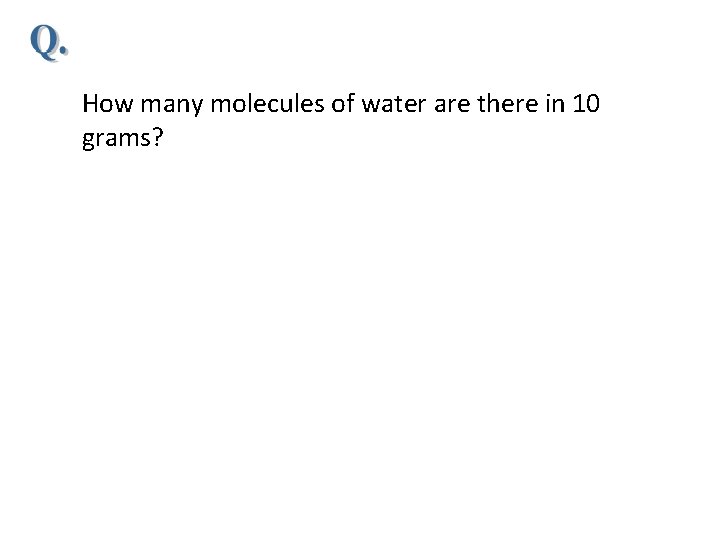
How many molecules of water are there in 10 grams?

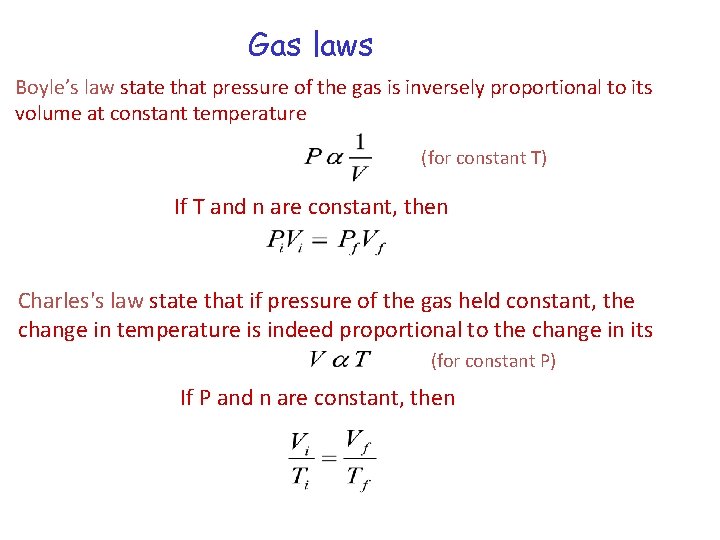
Gas laws Boyle’s law state that pressure of the gas is inversely proportional to its volume at constant temperature (for constant T) If T and n are constant, then Charles's law state that if pressure of the gas held constant, the change in temperature is indeed proportional to the change in its (for constant P) If P and n are constant, then

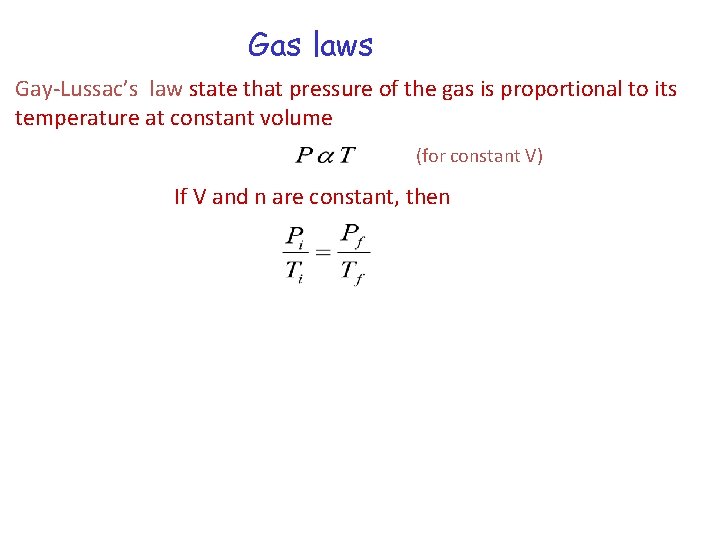
Gas laws Gay-Lussac’s law state that pressure of the gas is proportional to its temperature at constant volume (for constant V) If V and n are constant, then
![Ideal gas law Macroscopic form P Pa V m 3 n mol T K Ideal gas law (Macroscopic form) P [Pa] V [m 3] n [mol] T [K]](https://slidetodoc.com/presentation_image/1044a3fedd764ed1a96d6f825098d849/image-31.jpg)
Ideal gas law (Macroscopic form) P [Pa] V [m 3] n [mol] T [K] R = 8. 31 J / (mol K) (Microscopic form) P [Pa] V [m 3] N [particles] k = 1. 38 x 10 -23 J / K T [K]

A small bubble of air is released on the bottom of a column of beer. Assume that none of the air is absorbed by the water, and that the temperature is the same everywhere in the beer. (a) As the bubble rises, does its volume increase, decrease, or not change? (b) As the bubble rises, does the buoyant force on it increase, decrease, or not change?

An ideal gas that occupies 1. 2 m 3 at a pressure 1. 0 x 105 Pa and a temperature of 27 o. C is compressed to a volume of 0. 6 m 3 and heated to a temperature of 227 o. C. What is the new pressure?

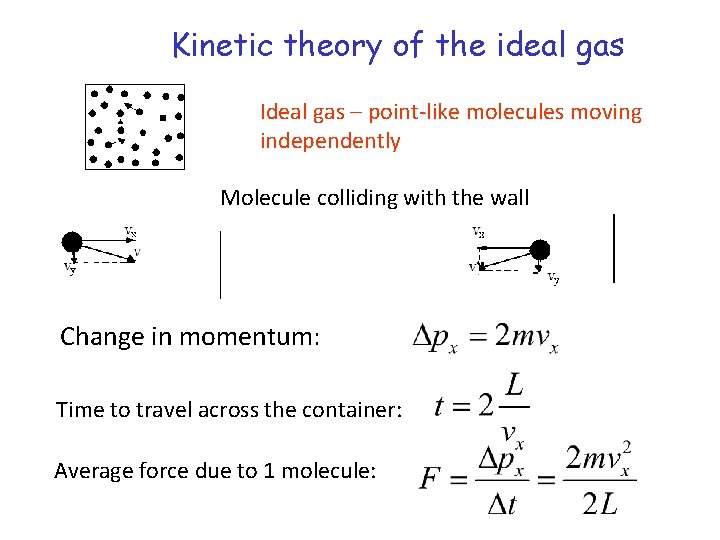
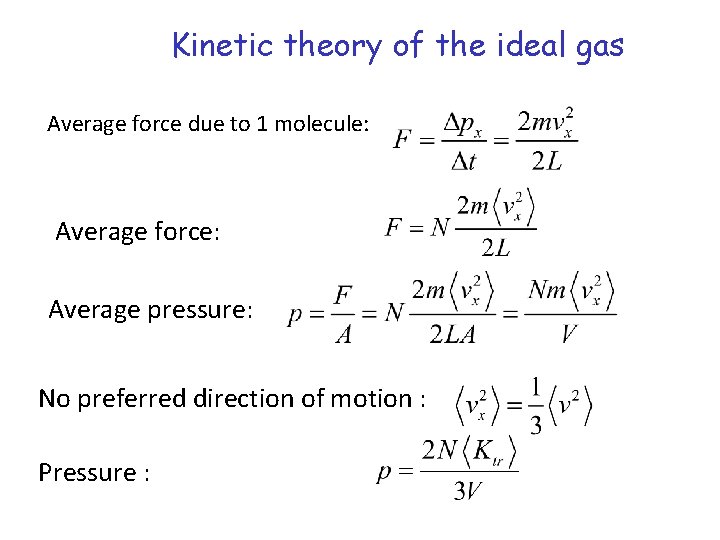
Kinetic theory of the ideal gas Ideal gas – point-like molecules moving independently Molecule colliding with the wall Change in momentum: Time to travel across the container: Average force due to 1 molecule:

Kinetic theory of the ideal gas Average force due to 1 molecule: Average force: Average pressure: No preferred direction of motion : Pressure :

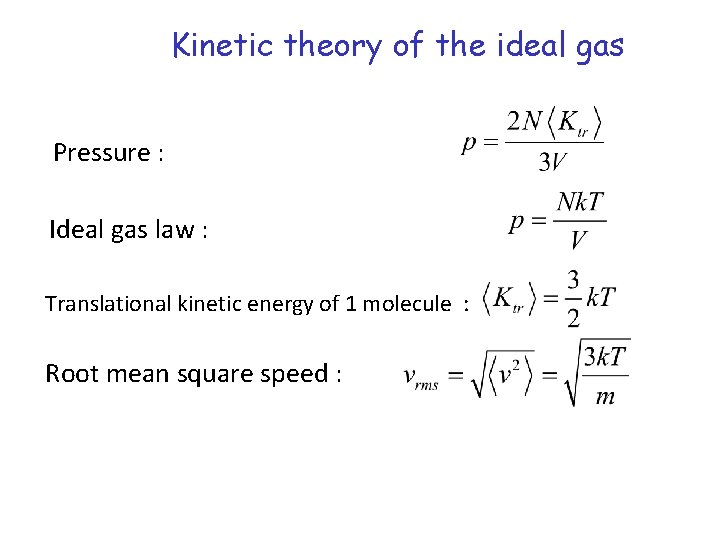
Kinetic theory of the ideal gas Pressure : Ideal gas law : Translational kinetic energy of 1 molecule : Root mean square speed :