To screen or not to screen for VRE
- Slides: 1

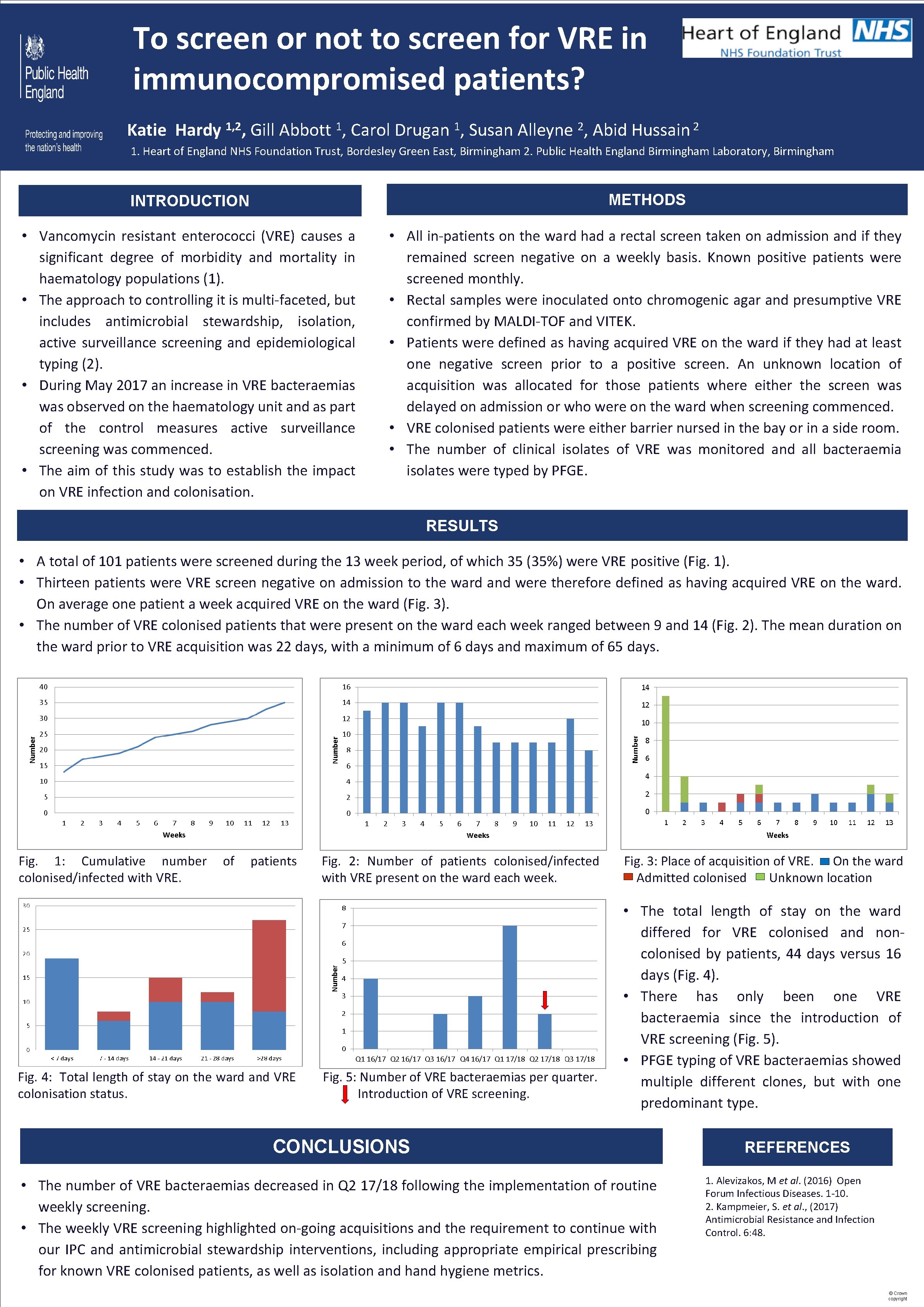
To screen or not to screen for VRE in immunocompromised patients? Katie Hardy 1, 2, Gill Abbott 1, Carol Drugan 1, Susan Alleyne 2, Abid 2 Hussain 1. Heart of England NHS Foundation Trust, Bordesley Green East, Birmingham 2. Public Health England Birmingham Laboratory, Birmingham INTRODUCTION METHODS • Vancomycin resistant enterococci (VRE) causes a significant degree of morbidity and mortality in haematology populations (1). • The approach to controlling it is multi-faceted, but includes antimicrobial stewardship, isolation, active surveillance screening and epidemiological typing (2). • During May 2017 an increase in VRE bacteraemias was observed on the haematology unit and as part of the control measures active surveillance screening was commenced. • The aim of this study was to establish the impact on VRE infection and colonisation. • All in-patients on the ward had a rectal screen taken on admission and if they remained screen negative on a weekly basis. Known positive patients were screened monthly. • Rectal samples were inoculated onto chromogenic agar and presumptive VRE confirmed by MALDI-TOF and VITEK. • Patients were defined as having acquired VRE on the ward if they had at least one negative screen prior to a positive screen. An unknown location of acquisition was allocated for those patients where either the screen was delayed on admission or who were on the ward when screening commenced. • VRE colonised patients were either barrier nursed in the bay or in a side room. • The number of clinical isolates of VRE was monitored and all bacteraemia isolates were typed by PFGE. RESULTS • A total of 101 patients were screened during the 13 week period, of which 35 (35%) were VRE positive (Fig. 1). • Thirteen patients were VRE screen negative on admission to the ward and were therefore defined as having acquired VRE on the ward. On average one patient a week acquired VRE on the ward (Fig. 3). • The number of VRE colonised patients that were present on the ward each week ranged between 9 and 14 (Fig. 2). The mean duration on the ward prior to VRE acquisition was 22 days, with a minimum of 6 days and maximum of 65 days. Fig. 1: Cumulative number colonised/infected with VRE. of patients Fig. 4: Total length of stay on the ward and VRE colonisation status. Fig. 2: Number of patients colonised/infected with VRE present on the ward each week. Fig. 5: Number of VRE bacteraemias per quarter. Introduction of VRE screening. Fig. 3: Place of acquisition of VRE. On the ward Admitted colonised Unknown location • The total length of stay on the ward differed for VRE colonised and noncolonised by patients, 44 days versus 16 days (Fig. 4). • There has only been one VRE bacteraemia since the introduction of VRE screening (Fig. 5). • PFGE typing of VRE bacteraemias showed multiple different clones, but with one predominant type. CONCLUSIONS • The number of VRE bacteraemias decreased in Q 2 17/18 following the implementation of routine weekly screening. • The weekly VRE screening highlighted on-going acquisitions and the requirement to continue with our IPC and antimicrobial stewardship interventions, including appropriate empirical prescribing for known VRE colonised patients, as well as isolation and hygiene metrics. REFERENCES 1. Alevizakos, M et al. (2016) Open Forum Infectious Diseases. 1 -10. 2. Kampmeier, S. et al. , (2017) Antimicrobial Resistance and Infection Control. 6: 48. © Crown copyright