To Err Is Human Not To Err Is












































- Slides: 64

To Err Is Human; Not To Err Is Better! Vaccination Errors and How to Prevent Them Deborah L. Wexler, MD Immunization Action Coalition deborah@immunize. org National Immunization Conference Dallas, Texas March 31, 2009

With thanks to Donna L. Weaver, RN, MN, National Center for Immunization and Respiratory Diseases, CDC and Teresa A. Anderson, DDS, MPH Consultant, Immunization Action Coalition

Types of vaccination errors • Storage and handling • Administration • Scheduling • Documentation

Vaccine storage and handling • Vaccines are fragile and must be kept at recommended temperatures at all times • It is better to NOT VACCINATE than to administer a dose of vaccine that has been mishandled. Adapted from CDC

The results of storage and handling errors • You can lose a lot of money (vaccines are costly!) • You must revaccinate anyone who received a dose of compromised vaccine, or, if the problem goes unnoticed, the patient remains susceptible to the disease. • You will have to explain to irate parents why their children must repeat the vaccine doses. • The media may find out and provide your CDC

Newspaper Headlines

This is the kind of publicity you don’t want “ 1, 900 doses of flu vaccine spoil in hospital’s faulty fridge” (West Allis, WI; 11/3/04) “Kaiser mishandles flu vaccine” (Fresno, CA; 12/15/04) “Storage errors cause thousands to be vaccinated again” (Knoxville, TN; 1/21/05) “U. S. doctor accused of giving last year’s flu vaccine” (Bellingham, WA; 11/6/04) “Frozen vaccine could cost state more than $30, 000” (Arkansas; 11/19/04)

How to avoid storage and handling • problems Assign a vaccine manager • Store vaccines appropriately • Monitor and record refrigerator and freezer temperatures twice daily and review the results twice a day • Use only certified calibrated thermometers • Maintain temperature logs for 3 years • Implement a vaccine emergency system • Take immediate action for out-of-range temperatures • Do not store anything else in the refrigerator Adapted CDC

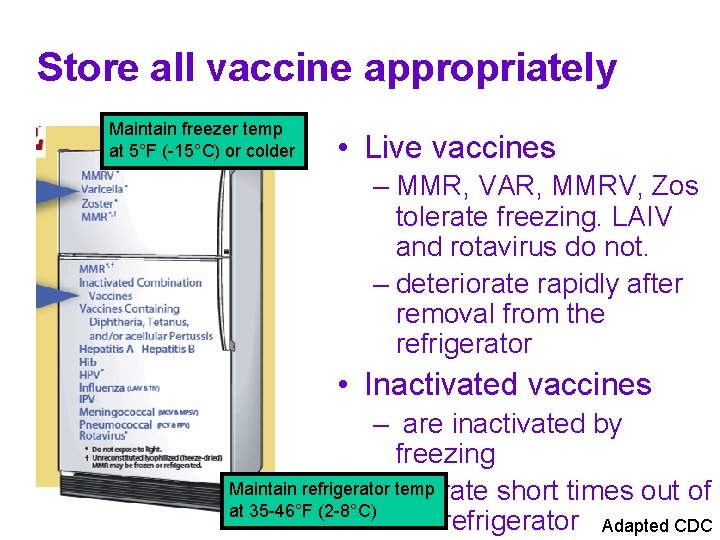
Store all vaccine appropriately Maintain freezer temp at 5°F (-15°C) or colder • Live vaccines – MMR, VAR, MMRV, Zos tolerate freezing. LAIV and rotavirus do not. – deteriorate rapidly after removal from the refrigerator • Inactivated vaccines – are inactivated by freezing Maintain refrigerator – temp tolerate short times out of at 35 -46°F (2 -8°C) the refrigerator Adapted CDC

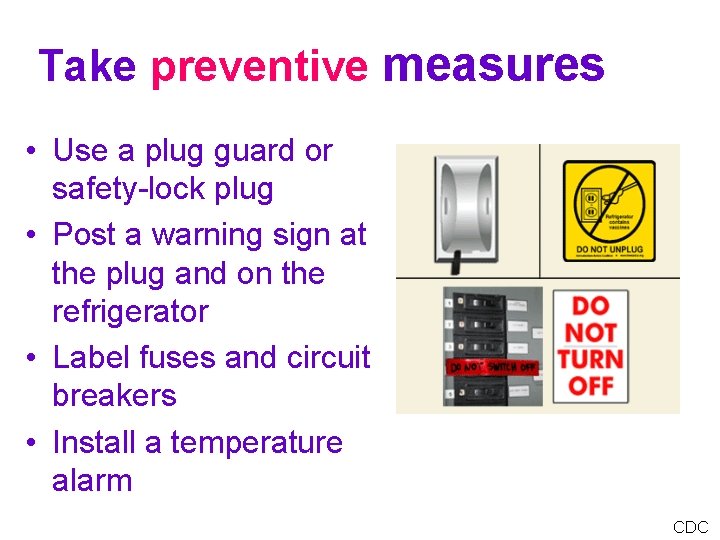
Take preventive measures • Use a plug guard or safety-lock plug • Post a warning sign at the plug and on the refrigerator • Label fuses and circuit breakers • Install a temperature alarm CDC

Vaccine handling basics • Open only one vial at a time • Store vaccine vials separate from other medications or biologics • Do not store vaccines on the door of the unit • Do not store food/beverages in refrigerator or freezer with vaccines • Keep light sensitive vaccines in their box until ready to use • Rotate your stocks so vaccines do not CDC

Pre-filling syringes • This practice is strongly discouraged by CDC • May result in vaccine administration errors • May consider in situations of heavy use of a single vaccine (e. g. , annual influenza clinic) • Consider using manufacturer-supplied prefilled syringes • Syringes other than those filled by manufacturer should be discarded at end of clinic day. Also, manufactured pre-filled syringes that have had the caps removed and a needle attached to the syringe should be discarded at the end of the day. Adapted CDC

Reconstituting Vaccines • Live virus vaccines and some inactivated vaccines must be administered promptly after reconstitution • If not administered within the time limit, these vaccinations need to be repeated! (If a live vaccine, with a 4 -week minimum interval. )

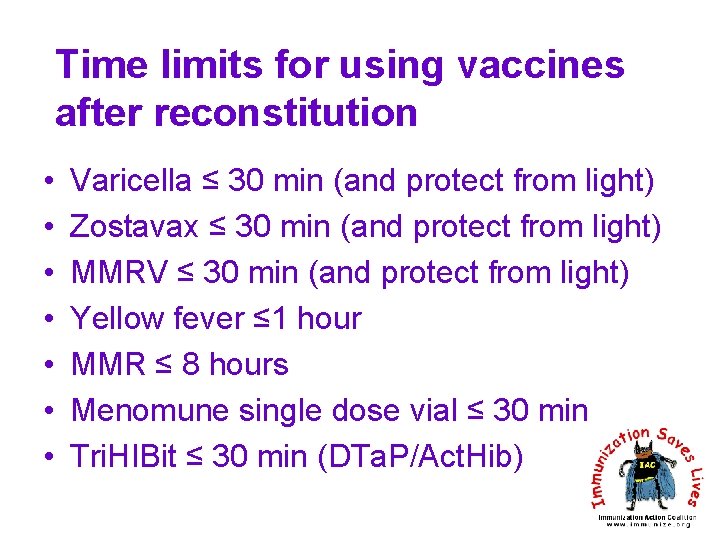
Time limits for using vaccines after reconstitution • • Varicella ≤ 30 min (and protect from light) Zostavax ≤ 30 min (and protect from light) MMRV ≤ 30 min (and protect from light) Yellow fever ≤ 1 hour MMR ≤ 8 hours Menomune single dose vial ≤ 30 min Tri. HIBit ≤ 30 min (DTa. P/Act. Hib)

Storage and handling resources from IAC – 1 • Checklist for Safe Vaccine Handling and Storage www. immunize. org/catg. d/p 3035. pdf • Don’t Be Guilty of These Errors in Vaccine Storage and Handling www. immunize. org/catg. d/p 3036. pdf • Vaccine Handling Tips www. immunize. org/catg. d/p 3048. pdf • Maintaining the Cold Chain During Transport www. immunize. org/catg. d/p 3049. pdf • Emergency Response Worksheet www. immunize. org/catg. d/p 3051. pdf

Storage and handling resources from IAC – 2 • Temperature Log for Vaccines (Fahrenheit) www. immunize. org/catg. d/p 3039. pdf • Temperature Log for Vaccines (Celsius) www. immunize. org/news. d/celsius. pdf • “Do Not Unplug” sign (color) www. immunize. org/news. d/2090 plugy. pdf • “Do Not Unplug” sign (black and white) www. immunize. org/news. d/2090 plug. pdf

Storage and handling resources from CDC’s comprehensive “Vaccine Storage and Handling Training Material Toolkit” can be accessed online at www 2 a. cdc. gov/vaccines/ed/shtoolkit

Storage and handling resources from CDC This 20 -page guide provides shipping requirements; condition upon arrival; storage requirements; shelf life; instructions for reconstitution and use; shelf life after reconstitution, thawing and opening; and any special instructions for all recommended vaccines. Go to: www. cdc. gov/vaccines/pubs/

Types of vaccination errors • Storage and handling • Administration • Scheduling • Documentation

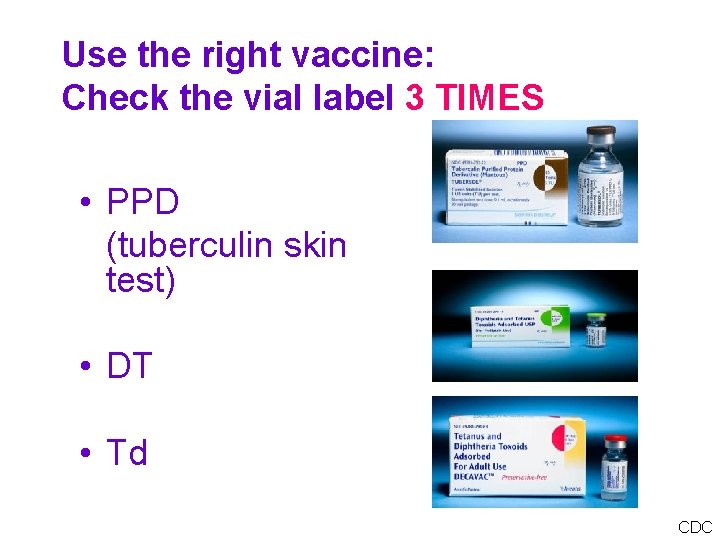
Types of Administration Errors • Wrong vaccine or wrong diluent • Wrong dosage • Expired vaccine • Wrong route / site / needle size

Use the right vaccine: Check the vial label 3 TIMES • PPD (tuberculin skin test) • DT • Td CDC

Diphtheria, tetanus, & pertussis vaccines • DTa. P (6 wks-6 yrs): Think of DTa. Peds • Tdap: Think of Adapted Talldap CDC

From our IAC email archive… HELP!

HELP! “A community health center in our area inadvertently gave a dose of Tdap to a 5 -year-old, instead of a DTa. P. What is their next best step to take under this circumstance? HELP! “Someone in our clinic gave DTa. P to a 50 -year-old instead of Tdap. How should this be handled? ”

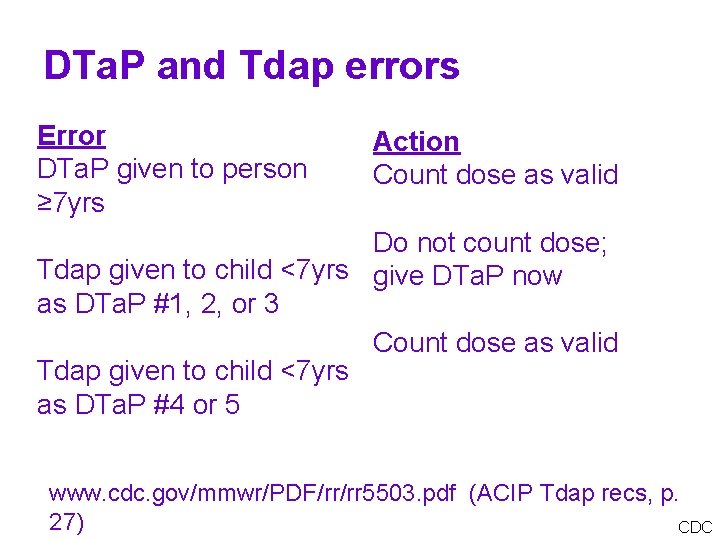
DTa. P and Tdap errors Error DTa. P given to person ≥ 7 yrs Action Count dose as valid Do not count dose; Tdap given to child <7 yrs give DTa. P now as DTa. P #1, 2, or 3 Count dose as valid Tdap given to child <7 yrs as DTa. P #4 or 5 www. cdc. gov/mmwr/PDF/rr/rr 5503. pdf (ACIP Tdap recs, p. 27) CDC

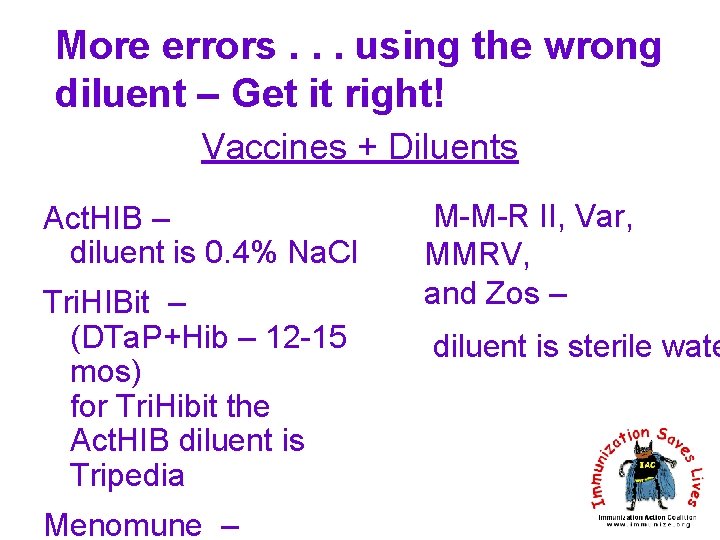
More errors. . . using the wrong diluent – Get it right! Vaccines + Diluents Act. HIB – diluent is 0. 4% Na. Cl Tri. HIBit – (DTa. P+Hib – 12 -15 mos) for Tri. Hibit the Act. HIB diluent is Tripedia Menomune – M-M-R II, Var, MMRV, and Zos – diluent is sterile wate

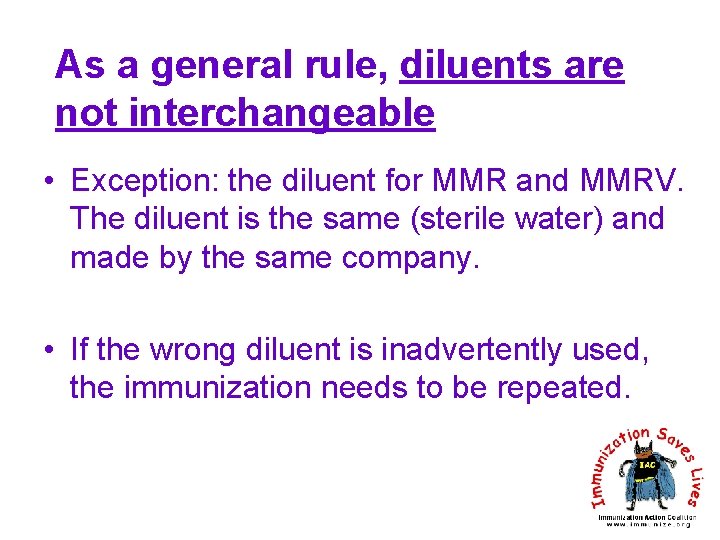
As a general rule, diluents are not interchangeable • Exception: the diluent for MMR and MMRV. The diluent is the same (sterile water) and made by the same company. • If the wrong diluent is inadvertently used, the immunization needs to be repeated.

Giving the wrong vaccine will rarely cause a serious problem, but… • Additional doses can lead to more vigorous local reactions • Patient may be left unprotected against disease • Additional cost • Inconvenience to patient/parent • May cause loss of faith in provider or complaint to state board

HELP! “Yesterday I took my two year old twins to the doctor for their check-up. They were given their vaccinations at that time. Today we received a phone call that one of the vaccinations they were given was the wrong one… I do not know if this will cause them any harm or if I should worry that down the road they may experience problems as a result of this mess-up. I am just wondering what I should do. I am planning to find someone else to care for my twins. It is scary that not only the doctor messed up the order, but the nurses that gave the vaccinations did not question it. ”

Another administration error: giving the wrong dose

HELP! One of our staff gave a dose of pediatric hepatitis A vaccine to an adult patient by mistake. How do we remedy this error? ANSWER If less than a full age-appropriate dose of any vaccine is given, the dose should not be counted. The person should be revaccinated with the appropriate dose as soon as possible.

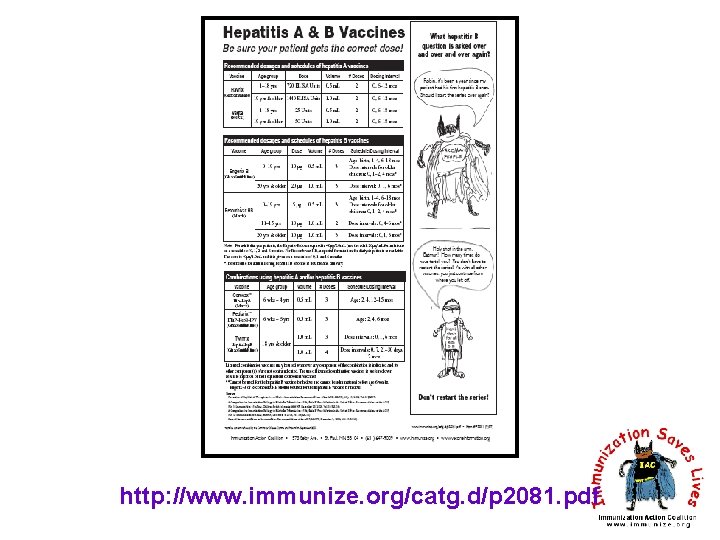
http: //www. immunize. org/catg. d/p 2081. pdf

The right dose: split or partial doses + • Split or partial (incomplete) doses are NOT valid doses – But the following DO count • LAIV if person sneezes • Rotavirus vaccine if infant regurgitates, spits out, or CDC vomits

The right dose: combining vaccines + • Vaccines should NEVER be combined in the same syringe unless FDA approved for this purpose CDC

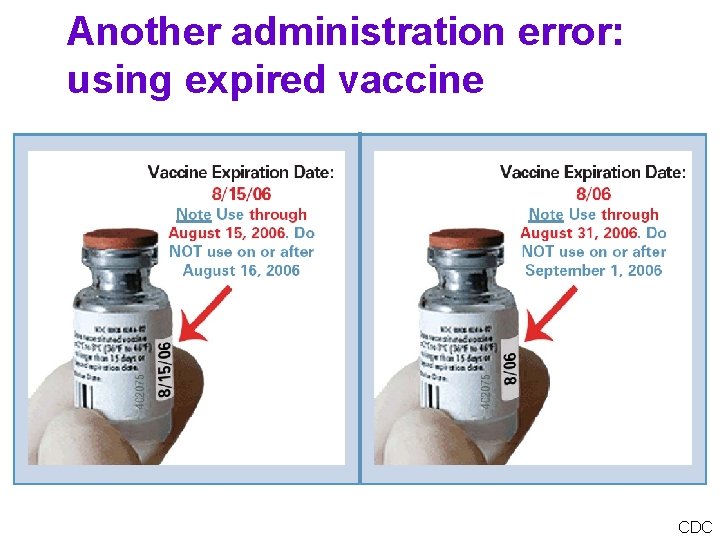
Another administration error: using expired vaccine CDC

HELP! “A physician just called and gave a child a dose of expired vaccine. I am assuming the dose should be readministered. Please advise. ” ANSWER The dose should be repeated. If the expired dose is a live virus vaccine (e. g. , MMR, Var) you must wait at least 4 weeks after the previous (expired) dose was given before repeating it. The repeat dose of an expired inactivated vaccine can be given on the same day or any other time.

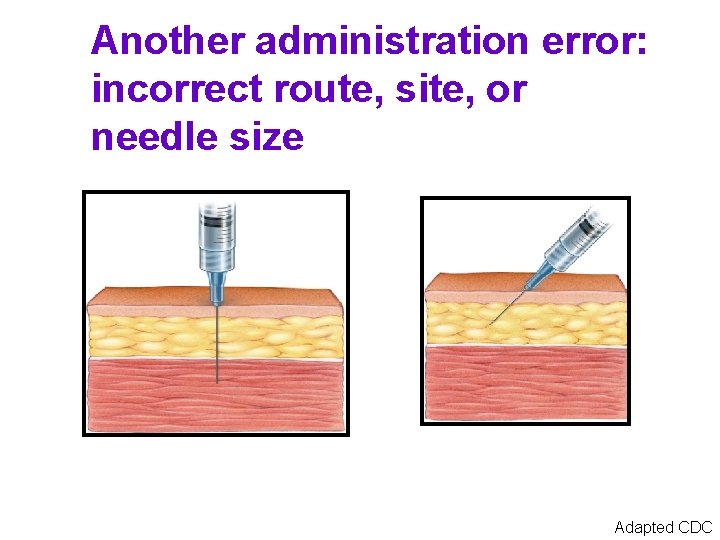
Another administration error: incorrect route, site, or needle size Adapted CDC

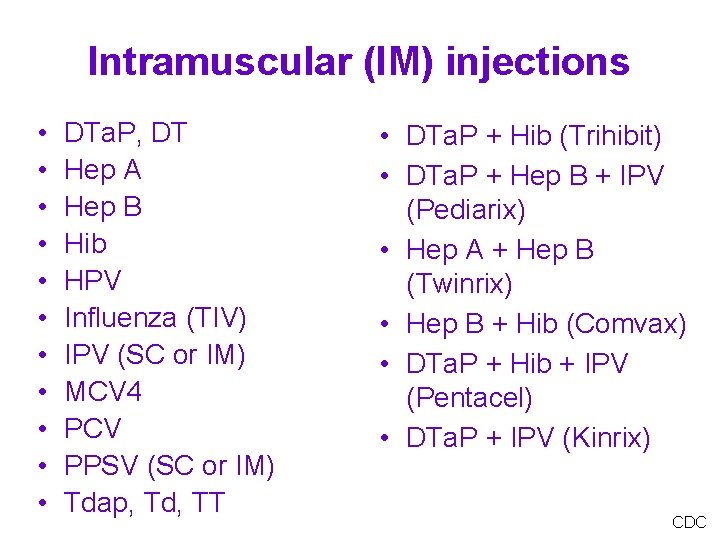
Intramuscular (IM) injections • • • DTa. P, DT Hep A Hep B Hib HPV Influenza (TIV) IPV (SC or IM) MCV 4 PCV PPSV (SC or IM) Tdap, Td, TT • DTa. P + Hib (Trihibit) • DTa. P + Hep B + IPV (Pediarix) • Hep A + Hep B (Twinrix) • Hep B + Hib (Comvax) • DTa. P + Hib + IPV (Pentacel) • DTa. P + IPV (Kinrix) CDC

Multiple vaccinations • Use thigh for multiple IM injections in infants and young children • The deltoid muscle can be used for children older than one year and adults • Separate each injection by at least 1” • Administer vaccine and immune globulin at separate sites • Combination vaccines can reduce the number of injections needed CDC

Administering multiple vaccinations www. cdc. gov/vaccines/pubs/pinkbook/downloads/appendice s/D/

HELP! “One of our nurses accidentally gave zoster vaccine IM instead of SC. Can you tell me what we need to do? ” ANSWER Vaccines should always be given by the route recommended by the manufacturer. If this does inadvertently happen, ACIP recommends that vaccines given by the wrong route be counted as valid with two exceptions: hepatitis B or rabies vaccine given by any route other than IM (and in the deltoid or anterolateral thigh muscle) should not be counted as valid and should be repeated.

Miscellaneous administration hints • Syncope occurs most often in adolescents and young adults; consider observing patients 15 -20 minutes after vaccination. • OSHA regulations do not require the wearing of gloves when administering vaccinations, unless the person administering the vaccine is likely to come into contact with potentially infectious body fluids or has an open lesion on their hand. • It is not recommended to change needles after a vaccine dose has been drawn into a syringe. • ACIP does not recommend aspiration when administering vaccines because no data exist

Vaccine administration resources from IAC • How to Administer Intramuscular (IM) Injections www. immunize. org/catg. d/p 2020. pdf • How to Administer IM and SC Injections to Adults www. immunize. org/catg. d/p 2020 a. pdf • Administering Vaccines to Adults: Dose, Route, Site, Needle Size, and Preparation www. immunize. org/catg. d/p 3084. pdf • Administering Vaccines: Dose, Route, Site, and Needle Size www. immunize. org/catg. d/p 3085. pdf

Vaccine administration resource from Calif. Dept. of Health Services "Immunization Techniques: Safe, Effective, Caring" is a 35 -minute DVD that teaches best practices about how to administer IM and SC vaccines. It is designed for use as a "hands-on" instructional program for staff The DVD discusses the following: www. immunize. org/sh • Anatomic sites op • Choice of needle size • Routes of administration • How to draw up doses of vaccine

Types of vaccination errors • Storage and handling • Administration • Scheduling • Documentation

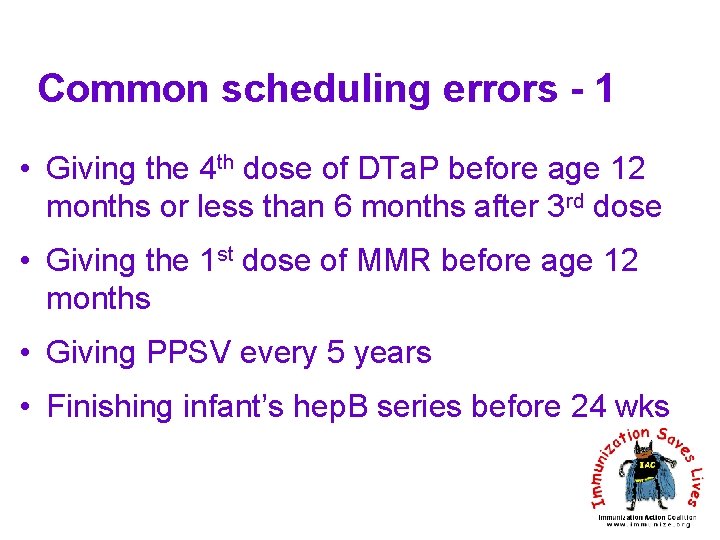
Common scheduling errors - 1 • Giving the 4 th dose of DTa. P before age 12 months or less than 6 months after 3 rd dose • Giving the 1 st dose of MMR before age 12 months • Giving PPSV every 5 years • Finishing infant’s hep. B series before 24 wks

Common scheduling errors - 2 • Giving Tri. HIBit (DTa. P / Act. Hib) at 2, 4, and 6 mos • Giving rotavirus vaccine after 8 months 0 days • Giving any vaccine (except hepatitis B) before age 6 weeks • Restarting any vaccine series because of a longer-than-recommended interval

IMPORTANT RULE: Vaccine doses should not be administered at intervals less than the recommended minimal intervals or earlier than the minimal ages. But, there is no maximum interval! (Except for oral typhoid vaccine in some circumstances. )

HELP! “I plan to visit China and stay a year. My doctor said I can have the first dose of hepatitis A vaccine right now and then get the second dose three months later. But from your website, I saw ‘second dose no sooner than 6 months after the first dose. ’ From CDC website I saw 6 -18 months after the first dose. From NIH website, I saw 6 months after the first dose. Who is correct? ” It might be embarrassing if your patients have to correct you! Know the minimum intervals and ages www. cdc. gov/vaccines/pubs/pinkbook/downloads/ appendices/A/age-interval-table. pdf

Know how to use the 4 -day “grace period” Since 2002, ACIP recommends that doses administered up to 4 days before the minimum interval or age can be counted as valid. The 4 -day "grace period" should not be used when scheduling future vaccination visits, or applied to the 28 -day interval between live parenteral vaccines of two different vaccines not administered at the same visit. It should be used primarily when reviewing vaccination records (for example, when evaluating a vaccination record prior to entry to daycare or school). Use of the grace period may create a conflict with state daycare or school entry vaccination requirements.

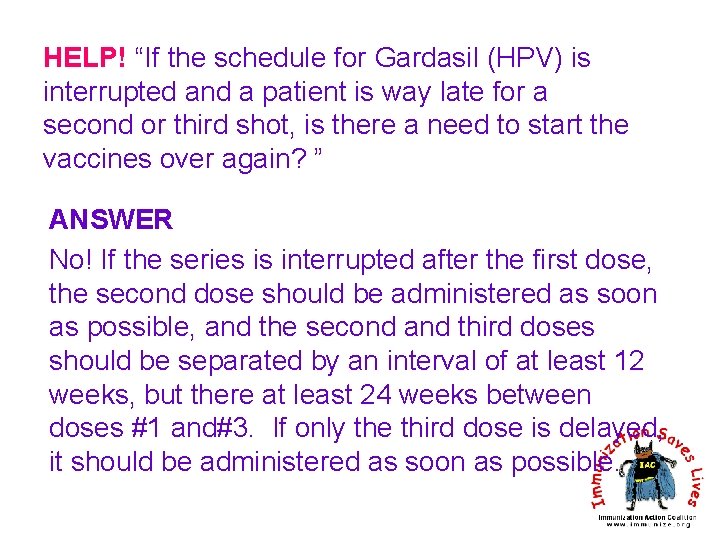
HELP! “If the schedule for Gardasil (HPV) is interrupted and a patient is way late for a second or third shot, is there a need to start the vaccines over again? ” ANSWER No! If the series is interrupted after the first dose, the second dose should be administered as soon as possible, and the second and third doses should be separated by an interval of at least 12 weeks, but there at least 24 weeks between doses #1 and#3. If only the third dose is delayed, it should be administered as soon as possible.

HELP! “How can we quickly determine how to catch up children who have fallen behind on their shots? ” ANSWER Infants or children who are more than 1 month or 1 dose behind schedule should be on an accelerated schedule, which means the intervals between doses should be reduced to the minimum allowable. Catch-up schedules for children and adolescents are included with each year's U. S. Recommended Immunization Schedule that is issued by ACIP, AAP, and AAFP.

Scheduling resources from IAC • Summary of Recommendations for Childhood and Adolescent Immunization www. immunize. org/childrules • Summary of Recommendations for Adult Immunization www. immunize. org/adultrules • Pneumococcal Polysaccharide Vaccine (PPSV): CDC answers your questions www. immunize. org/catg. d/2015 pne. pdf

Where to get immunization schedules • Official child and adult IZ schedules on CDC website in various sizes (pocket to 11”x 17”), in color and B & W, and for office or commercial press printers at www. cdc. gov/vaccines/recs/schedules. • IAC sells 6 -page laminated 8. 5”x 11” versions of the child/teen and adult schedules that include a list of contraindications and precautions www. immunize. org/shop • Society of Teachers of Family Medicine has schedules that can be downloaded to a Palm OS Handheld. Go to www. immunizationed. org/Any. Page. aspx? pgid=2

Types of vaccination errors • Storage and handling • Administration • Scheduling • Documentation

Types of documentation errors • Not providing VISs • Not knowing if written consent is required • Not recording all needed information

HELP! “My 2 month old child was recently inoculated at his pediatrician’s office. The day following the immunizations my son spiked a high fever and I was extremely concerned. I called our local hospital and found out that I should have been given a VIS sheet for each of the inoculations that my child received. I did bring this matter up with the pediatrician’s office and I was told by the office manager that she didn’t know of any law that mandated they give information sheets out… My question is to whom do I report this incident to? I no longer take my child to their office, but I want them to start doing things right. ” A minor side effect becomes a big problem because the parent wasn’t given a VIS…

HELP! “For a child, do we have the parent sign each time we give a vaccine in a series or is it enough to have them sign for the first one? ” ANSWER There is no federal law requiring written consent to vaccines. VISs cover both benefits and risks associated with vaccinations and they provide enough information that anyone reading them should be adequately informed. However, a few states have informed consent laws covering either procedural requirements (e. g. , whether consent may be oral or must be written) or substantive requirements (e. g. , types of information required). Check with your state immunization program.

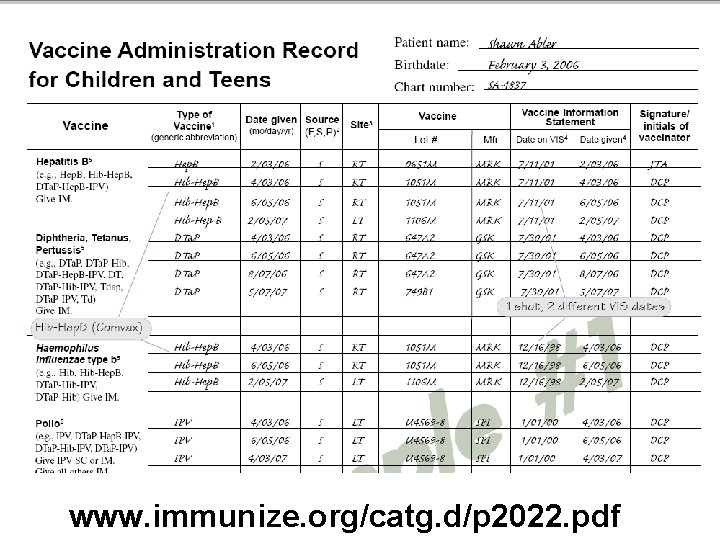
Required information to document • Type of vaccine e. g. , (MMR-V) (DTa. P-Hib) • Date vaccine is administered • Vaccine source (Federally- or State-supported or private) • Site given (RA, LA, RT, LT, IN, PO) • Vaccine lot # • Manufacturer • Date of the VIS • Date the VIS was given • Signature/initials of vaccinator

www. immunize. org/catg. d/p 2022. pdf

How to avoid vaccine errors… HELP! HELP!

Keep yourself current! • Read CDC’s “Pink Book” www. cdc. gov/vaccines/pubs/pinkbook/pinkchapters. htm • Find answers in relevant ACIP recommendations www. immunize. org/acip • Read IAC’s “Ask the Experts” Q&As www. immunize. org/askexperts • Subscribe to IAC Express for weekly updates www. immunize. org/subscribe

Need more help? • Email CDC’s experts: nipinfo@cdc. gov • Contact your vaccine rep or call the manufacturer • Call your state immunization manager -contact information can be found at www. immunize. org/coordinators • Email IAC: admin@immunize. org

The End