To Do n OWL n n Lab n

To Do… n OWL n n Lab n n Chapter 14 due Tuesday, April 24 Exam 3 review due Wednesday, April 25 Exam 3 n Thursday, April 26 1

Exam 3 Thursday, April 26, 7: 00 pm– 8: 30 pm; Rooms on the website (same). n Conflict: April 26; 5: 00 pm – 6: 30 pm in 1022 Lincoln Hall; sign up in 1026 CA n Practice exam will be posted online. n See me right away if you have a conflict with the exam and conflict times. n 2

Review Sessions n Tuesday, April 24 n n 165 Noyes Lab 7: 00 -9: 00 pm

Final Exam n n Friday, May 11, 8: 00 am – 11: 00 am 100 Noyes Lab Hour exams (this semester’s and practice exams) will be posted. There is no conflict exam scheduled. n n See me for University-sanctioned conflicts. We will discuss the Final Exam during lecture on Tuesday, May 1. 4
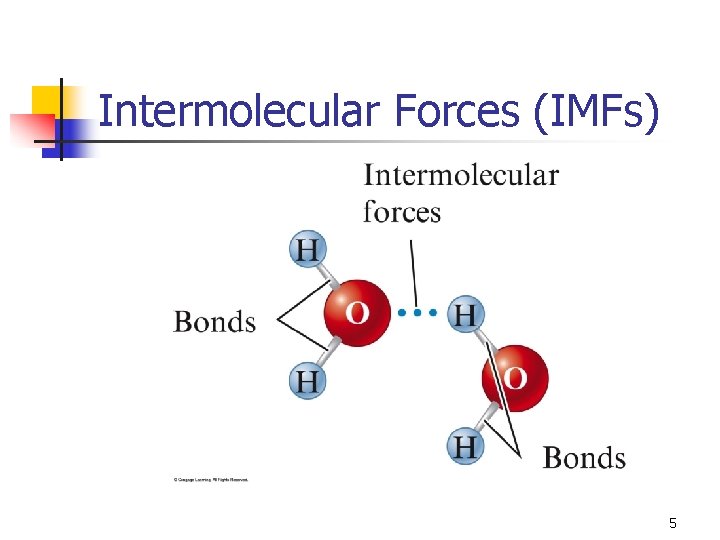
Intermolecular Forces (IMFs) 5
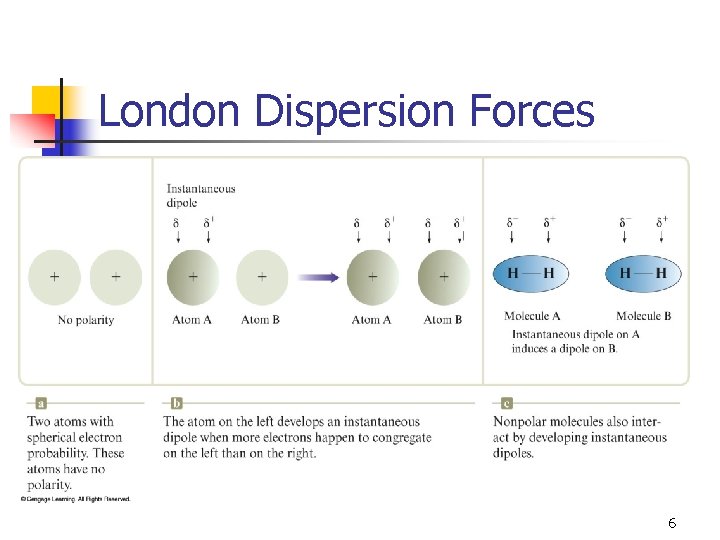
London Dispersion Forces 6

London dispersion forces • • • All molecules exhibit these forces. Due to temporary and induced dipoles. Strongest IMFs for non-polar molecules Examples include nitrogen (N 2) and methane (CH 4). With more electrons, more chance of an induced dipole. 7
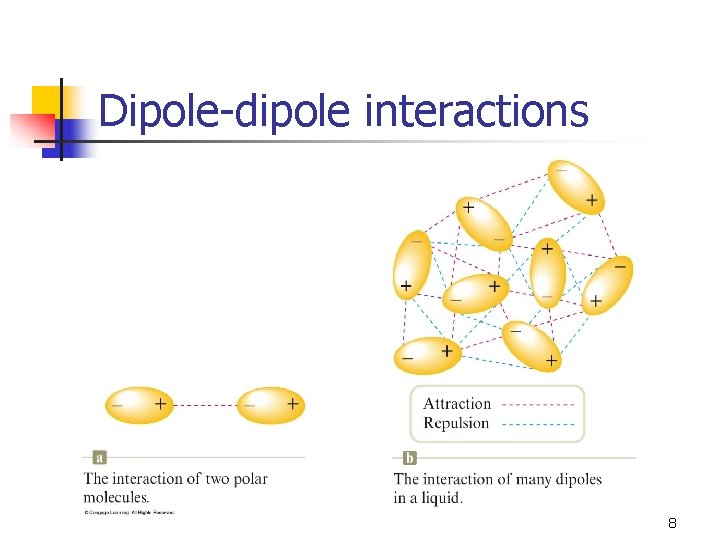
Dipole-dipole interactions 8

Dipole-dipole interactions • Polar molecules exhibit these forces. • • (also exhibit LDFs) Due to permanent dipoles. Stronger IMFs for than LDFs (given similar sized molecules). Some polar molecules (such as H 2 O) exhibit a special kind of dipole-dipole interaction. 9
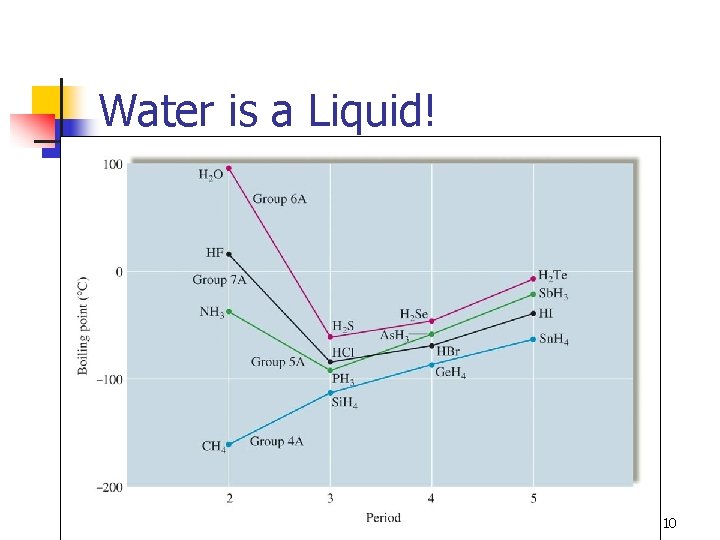
Water is a Liquid! 10
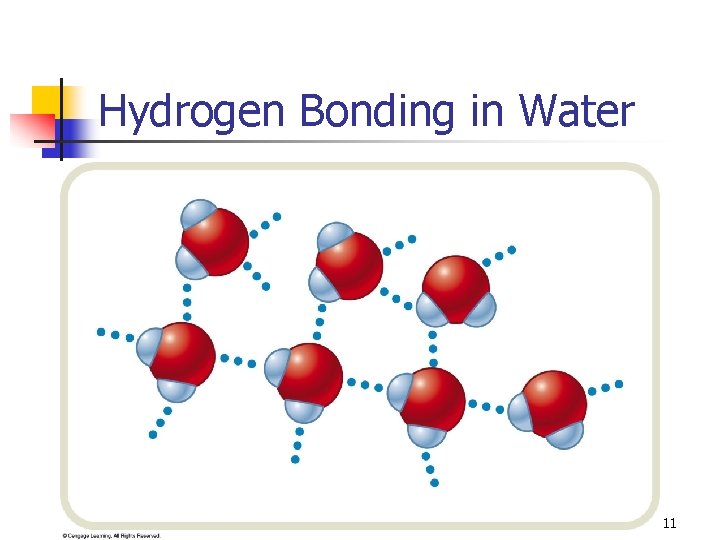
Hydrogen Bonding in Water 11
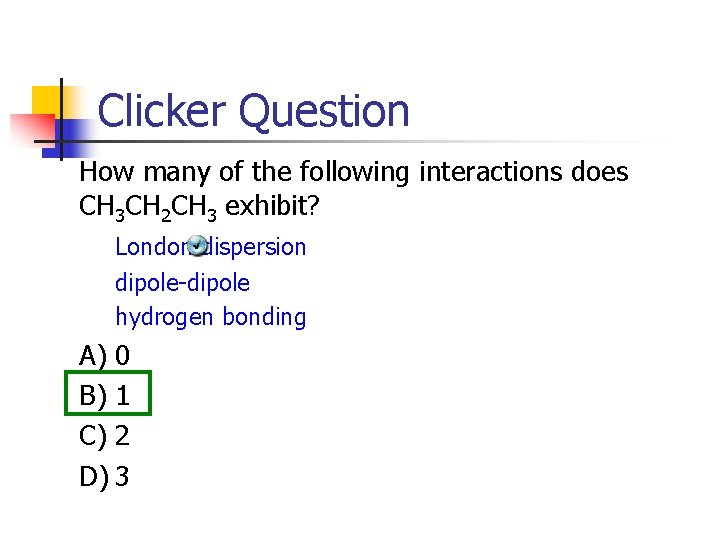
Clicker Question How many of the following interactions does CH 3 CH 2 CH 3 exhibit? London dispersion dipole-dipole hydrogen bonding A) 0 B) 1 C) 2 D) 3
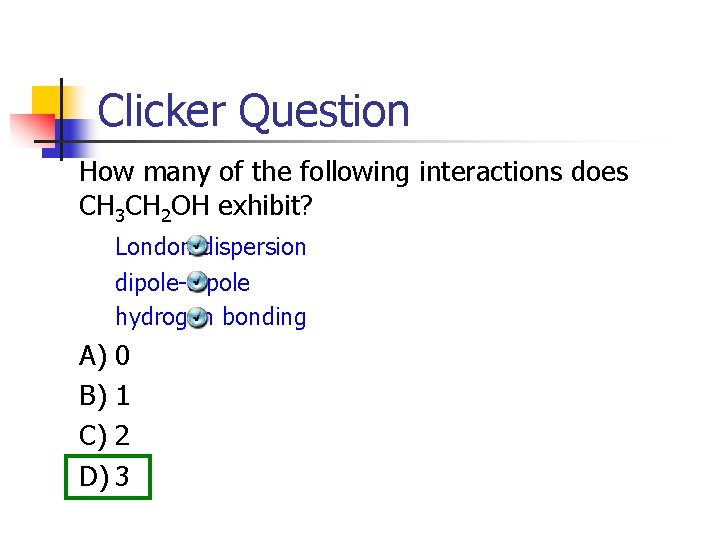
Clicker Question How many of the following interactions does CH 3 CH 2 OH exhibit? London dispersion dipole-dipole hydrogen bonding A) 0 B) 1 C) 2 D) 3
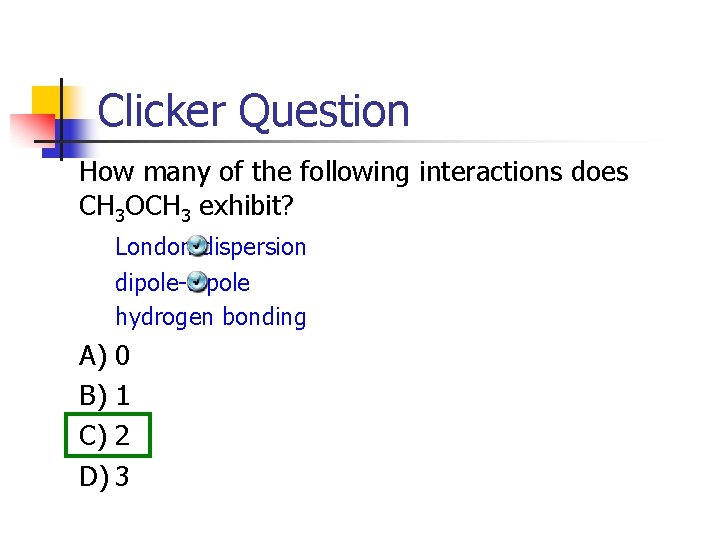
Clicker Question How many of the following interactions does CH 3 OCH 3 exhibit? London dispersion dipole-dipole hydrogen bonding A) 0 B) 1 C) 2 D) 3
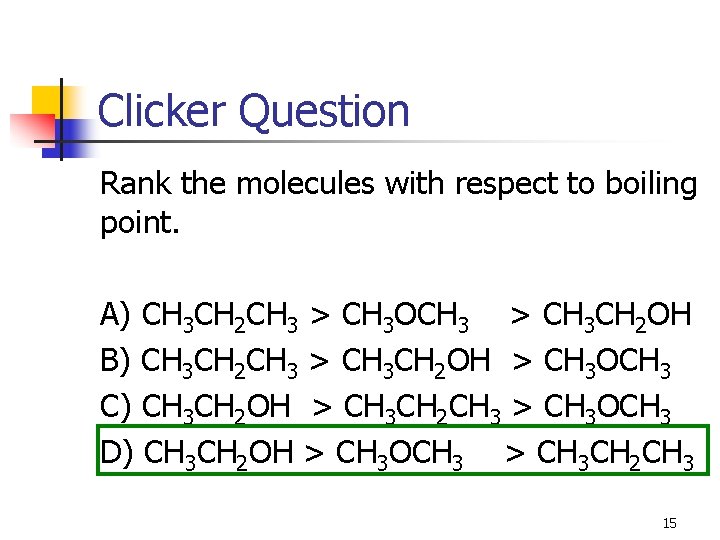
Clicker Question Rank the molecules with respect to boiling point. A) CH 3 CH 2 CH 3 > CH 3 OCH 3 > CH 3 CH 2 OH B) CH 3 CH 2 CH 3 > CH 3 CH 2 OH > CH 3 OCH 3 C) CH 3 CH 2 OH > CH 3 CH 2 CH 3 > CH 3 OCH 3 D) CH 3 CH 2 OH > CH 3 OCH 3 > CH 3 CH 2 CH 3 15
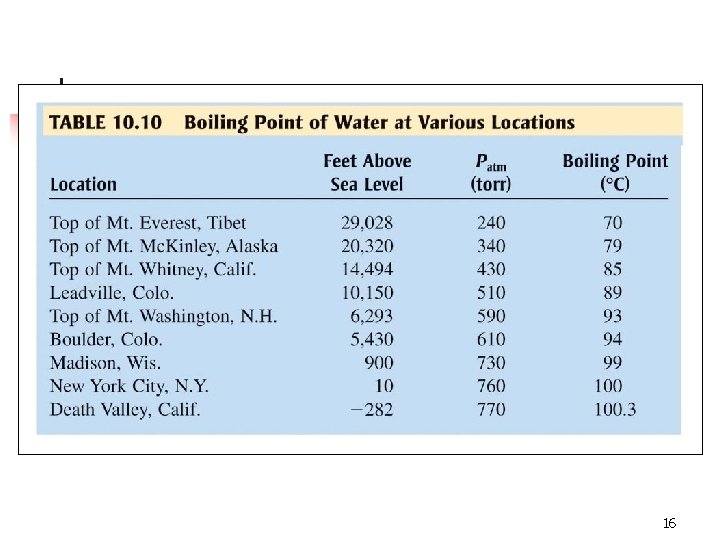
16

Ion-ion interactions • • Ionic compounds exhibit these forces. Due to ions. Strongest of the IMFs (given similar sized molecules). Example includes sodium chloride (Na. Cl). 17
- Slides: 17