Titrations Titrations Titration adding a known amount of







- Slides: 10

Titrations!

Titrations Titration: adding a known amount of solution of known concentration to a solution with an unknown concentration Goal: To determine the unknown concentration of a solution

Titrations Endpoint: the point of neutralization in a titration How do we know we reached the endpoint in a titration? We use an indicator and look for a color change! http: //www. youtube. com/watch? v=g 8 jd. CWC 10 v. Q

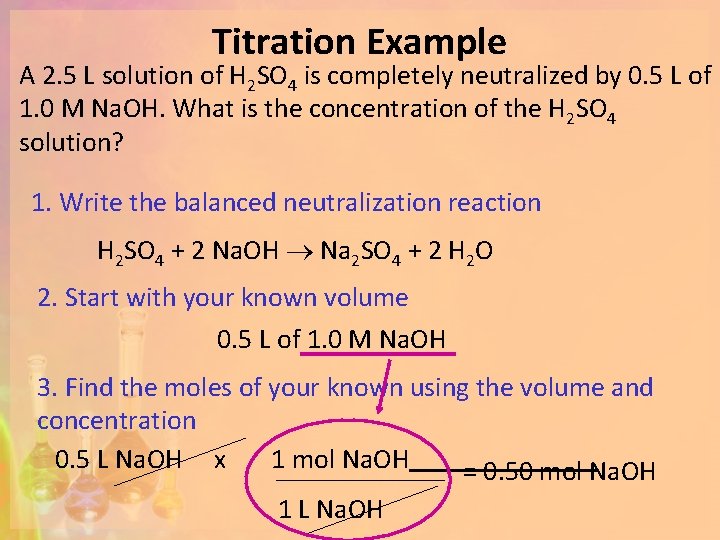
Titration Example A 2. 5 L solution of H 2 SO 4 is completely neutralized by 0. 5 L of 1. 0 M Na. OH. What is the concentration of the H 2 SO 4 solution? 1. 0 M Na. OH Show your work for each step: 1. Write the balanced neutralization reaction 2. Start with the volume of the known 3. Find the moles of your known using the volume and concentration 4. Use the molar ratio (the coefficients) to determine the unknown number of moles 5. Divide the moles by the volume to get concentration OR convert to grams ? M H 2 SO 4

Titration Example A 2. 5 L solution of H 2 SO 4 is completely neutralized by 0. 5 L of 1. 0 M Na. OH. What is the concentration of the H 2 SO 4 solution? 1. Write the balanced neutralization reaction H 2 SO 4 + 2 Na. OH Na 2 SO 4 + 2 H 2 O 2. Start with your known volume 0. 5 L of 1. 0 M Na. OH 3. Find the moles of your known using the volume and concentration 0. 5 L Na. OH x 1 mol Na. OH = 0. 50 mol Na. OH 1 L Na. OH

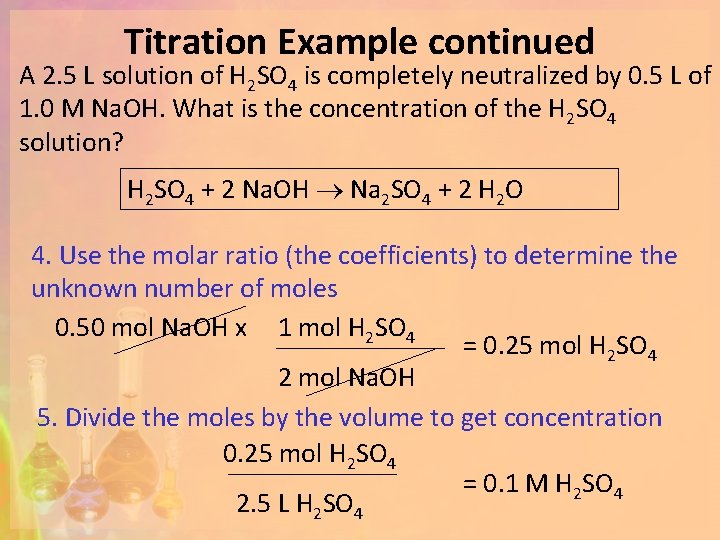
Titration Example continued A 2. 5 L solution of H 2 SO 4 is completely neutralized by 0. 5 L of 1. 0 M Na. OH. What is the concentration of the H 2 SO 4 solution? H 2 SO 4 + 2 Na. OH Na 2 SO 4 + 2 H 2 O 4. Use the molar ratio (the coefficients) to determine the unknown number of moles 0. 50 mol Na. OH x 1 mol H 2 SO 4 = 0. 25 mol H 2 SO 4 2 mol Na. OH 5. Divide the moles by the volume to get concentration 0. 25 mol H 2 SO 4 = 0. 1 M H 2 SO 4 2. 5 L H 2 SO 4

Titration Practice A 1. 5 L solution of HCl is completely neutralized by 2. 0 L of 0. 5 M Al(OH)3. What is the concentration of the HCl solution? 0. 5 M Al(OH)3 Show your work for each step: 1. Write the balanced neutralization reaction 2. Start with your known volume or mass 3. Find the moles of your known using the volume and concentration 4. Use the molar ratio (the coefficients) to determine the unknown number of moles 5. Divide the moles by the volume to get concentration OR convert to grams ? M HCl


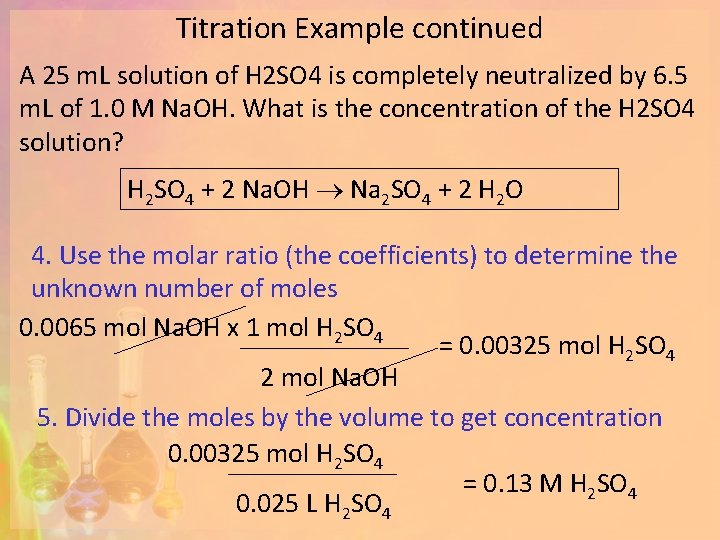
Example #2 A 25 m. L solution of H 2 SO 4 is completely neutralized by 6. 5 m. L of 1. 0 M Na. OH. What is the concentration of the H 2 SO 4 solution? 1. Write the balanced neutralization reaction H 2 SO 4 + 2 Na. OH Na 2 SO 4 + 2 H 2 O 2. Start with your known value 0. 0065 L of 1. 0 M Na. OH 3. Find the moles of your known using the volume and concentration 0. 0065 L Na. OH x 1 mol Na. OH = 0. 0065 mol Na. OH 1 L Na. OH

Titration Example continued A 25 m. L solution of H 2 SO 4 is completely neutralized by 6. 5 m. L of 1. 0 M Na. OH. What is the concentration of the H 2 SO 4 solution? H 2 SO 4 + 2 Na. OH Na 2 SO 4 + 2 H 2 O 4. Use the molar ratio (the coefficients) to determine the unknown number of moles 0. 0065 mol Na. OH x 1 mol H 2 SO 4 = 0. 00325 mol H 2 SO 4 2 mol Na. OH 5. Divide the moles by the volume to get concentration 0. 00325 mol H 2 SO 4 = 0. 13 M H 2 SO 4 0. 025 L H 2 SO 4