Titrations 1 Learning outcomes You should be able
Titrations 1

Learning outcomes You should be able to: Øbe able to carry out titrations and the associated calculations

3

Titration • Titration using a known concentration to find the concentration of an unknown • Today we will the concentration of an UNKNOWN acid by titrating it with a known base concentration: Na. OH = 0. 50 mol. dm-3 4

Stoichiometry The stoichiometry of an acid-base neutralization reaction is the same as that of any other reaction For example, in the reaction: Na. OH (aq) + HCl (aq) Na. Cl (aq) + H 2 O (l) 1 mol of Na. OH neutralizes 1 mol of HCl: 5

The p. H meter measures the p. H of the acid solution in the beaker as a solution of a base with a known concentration is added from the buret. 6

p. H curve for Strong Acids and Bases This abrupt change in p. H occurs at the equivalence point 7
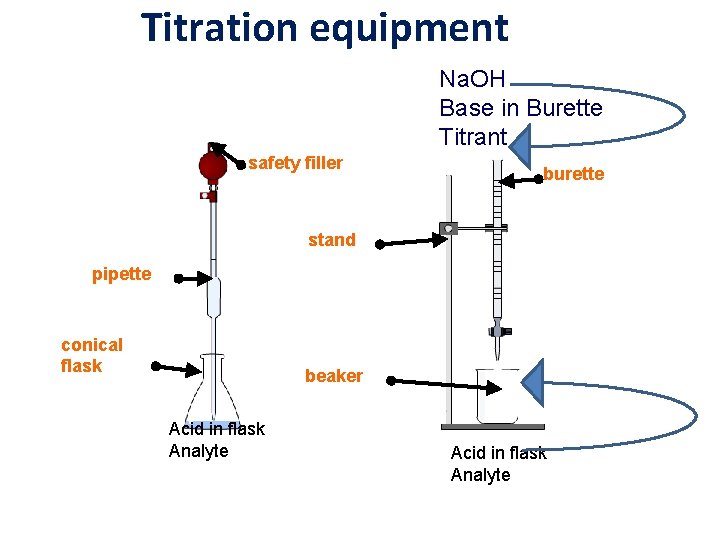
Titration equipment Na. OH Base in Burette Titrant safety filler burette stand pipette conical flask beaker Acid in flask Analyte

Titration with an Indicator 9

What’s the Point of a Titration Again? • To find the unknown concentration of an acid or a base. • Note the volume you started with and how much volume of the titrant you added 10

Practical 1. Set up equipment 2. Put one spatula of metal oxide and one spatula of carbon into your crucible and mix well. 3. Heat in a blue Bunsen flame until you see a change. 4. Leave your crucible to cool. SAFETY: Wear safety goggles. Wash hands after practical.
- Slides: 11