Titration Virtual Lab Note Get a copy of

Titration Virtual Lab

Note: Get a copy of the lab sheet for this lab from your teacher or from the class website. Read the procedure of the lab. Then look at the following slides to attain your data for the lab.
Step 1 • Be sure to create a neat, organized data table using a ruler to make straight lines.

Step 2 • The picture to the right shows the measurement of the vinegar in a 10 -m. L graduated cylinder. Be sure to record the volume to the nearest 0. 1 m. L.
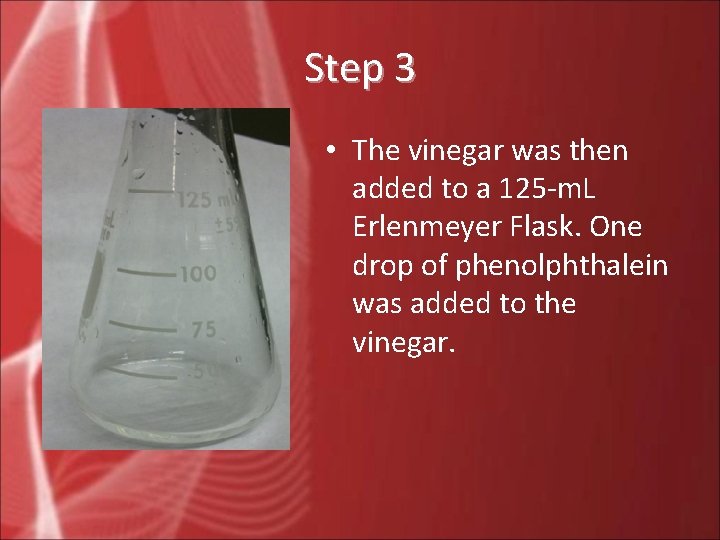
Step 3 • The vinegar was then added to a 125 -m. L Erlenmeyer Flask. One drop of phenolphthalein was added to the vinegar.

Step 4 • 0. 20 M Na. OH was added to a buret. Notice that the volume is not initially exactly on 0. 00 m. L. That does not matter. Record the exact initial volume for this trial to the nearest 0. 01 m. L.

Step 5 • A magnetic stirrer was not used in this lab.

Step 6 • The titration was carried out by adding small amounts of 0. 20 M Na. OH from the buret to the vinegar in the Erlenmeyer flask.

Step 7 • The endpoint was reached when the color of the phenolphthalein in the vinegar changed from colorless to light pink.

Step 8 • This is the buret at the end of the titration. Record the final volume of 0. 20 M Na. OH to the nearest 0. 01 m. L on your data table.

Step 9 • All equipment was cleaned and set up for trial 2. The pictures for your data collection of trial 2 are shown in the following slides.
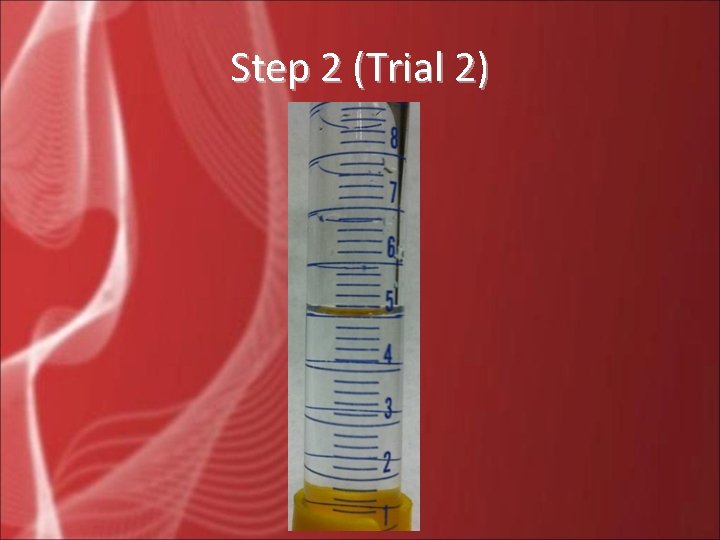
Step 2 (Trial 2)

Step 3 (Trial 2)

Step 4 (Trial 2)

Step 7 (Trial 2)

Step 8 (Trial 2)

Due Date You have two days for each day you were absent to complete the lab sheet for this lab. You need to perform all calculations showing all of your work and answer the questions in complete sentences. If you have any questions, see your chemistry teacher for help.
- Slides: 17