Titration standard solution u Titration Analytical method in



- Slides: 23

Titration standard solution u Titration • Analytical method in which a standard solution is used to determine the concentration of an unknown solution Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Titration u Equivalence point (endpoint) • Point at which equal amounts of H 3 O+ and OH- have been added. • Determined by… • indicator color change • dramatic change in p. H Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

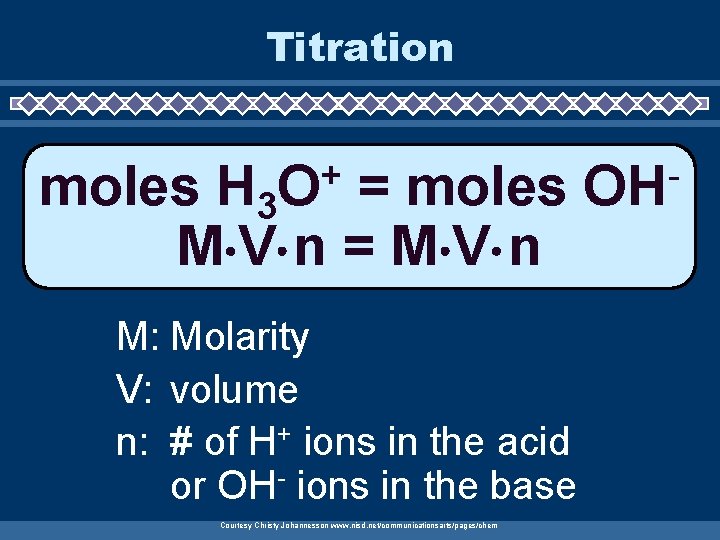
Titration + O moles H 3 = moles M V n = M V n M: Molarity V: volume n: # of H+ ions in the acid or OH- ions in the base Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem OH

Titration u 42. 5 m. L of 1. 3 M KOH are required to neutralize 50. 0 m. L of H 2 SO 4. Find the molarity of H 2 SO 4. H 3 O + M=? V = 50. 0 m. L n=2 OHM = 1. 3 M V = 42. 5 m. L n=1 MV# = MV# M(50. 0 m. L)(2) =(1. 3 M)(42. 5 m. L)(1 ) M = 0. 55 M H 2 SO 4 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

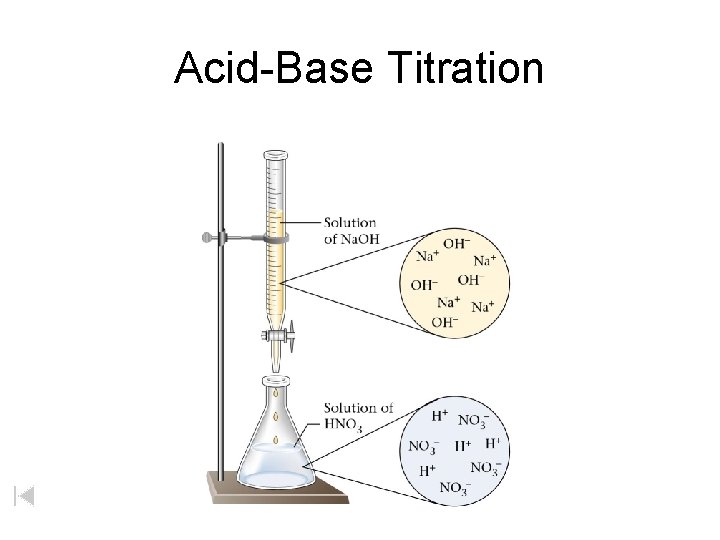
Acid-Base Titration

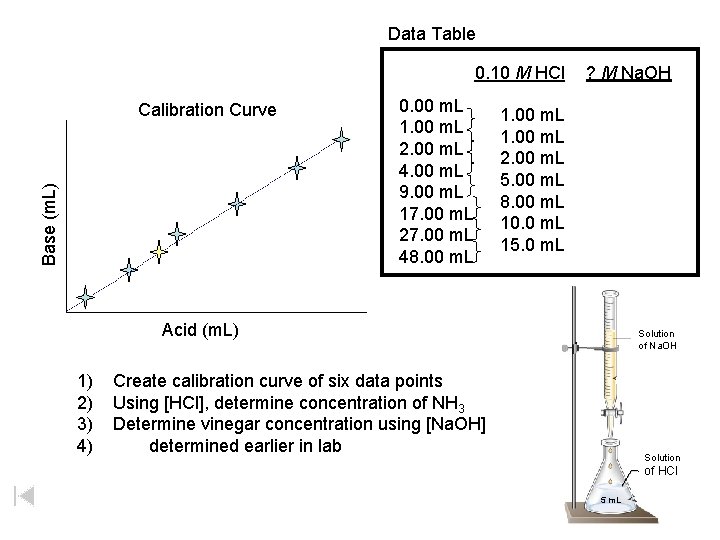
Data Table 0. 10 M HCl Base (m. L) Calibration Curve 0. 00 m. L 1. 00 m. L 2. 00 m. L 4. 00 m. L 9. 00 m. L 17. 00 m. L 27. 00 m. L 48. 00 m. L ? M Na. OH 1. 00 m. L 2. 00 m. L 5. 00 m. L 8. 00 m. L 10. 0 m. L 15. 0 m. L Acid (m. L) 1) 2) 3) 4) Solution of of Na. OH Create calibration curve of six data points Using [HCl], determine concentration of NH 3 Determine vinegar concentration using [Na. OH] determined earlier in lab Solution of HCl 5 m. L

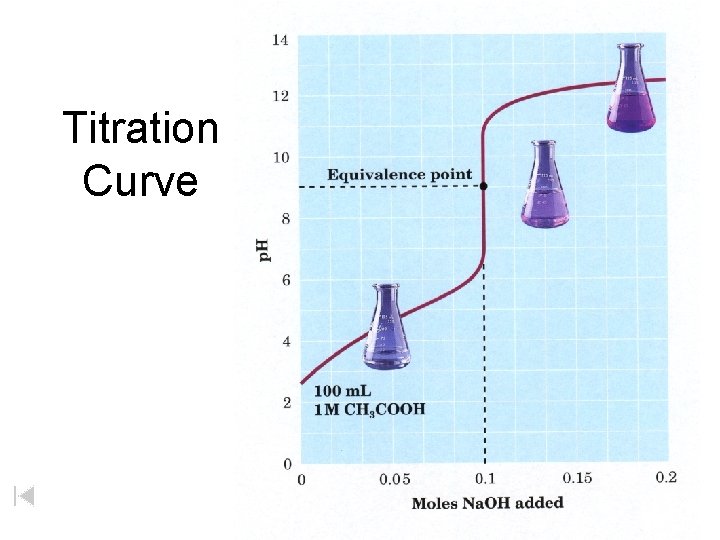
Titration Curve

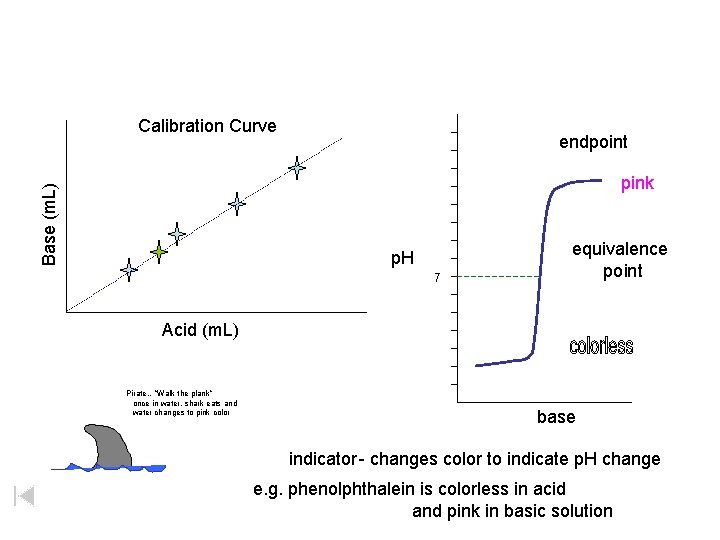
Titration indicator -changes color to indicate p. H change e. g. phenolpthalein is colorless in acid and pink in basic solution endpoint pink equivalence point p. H 7 Pirate…”Walk the plank” once in water, shark eats and water changes to pink color base

Calibration Curve endpoint Base (m. L) pink p. H 7 equivalence point Acid (m. L) Pirate…”Walk the plank” once in water, shark eats and water changes to pink color base indicator - changes color to indicate p. H change e. g. phenolphthalein is colorless in acid and pink in basic solution

Calibration Curve endpoint Base (m. L) pink p. H 7 equivalence point Acid (m. L) Pirate…”Walk the plank” once in water, shark eats and water changes to pink color base indicator - changes color to indicate p. H change e. g. phenolphthalein is colorless in acid and pink in basic solution

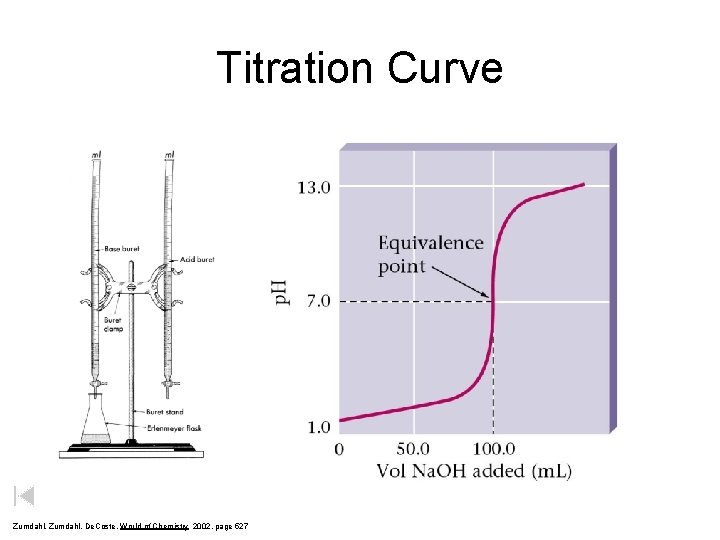
Titration Curve Zumdahl, De. Coste, World of Chemistry 2002, page 527

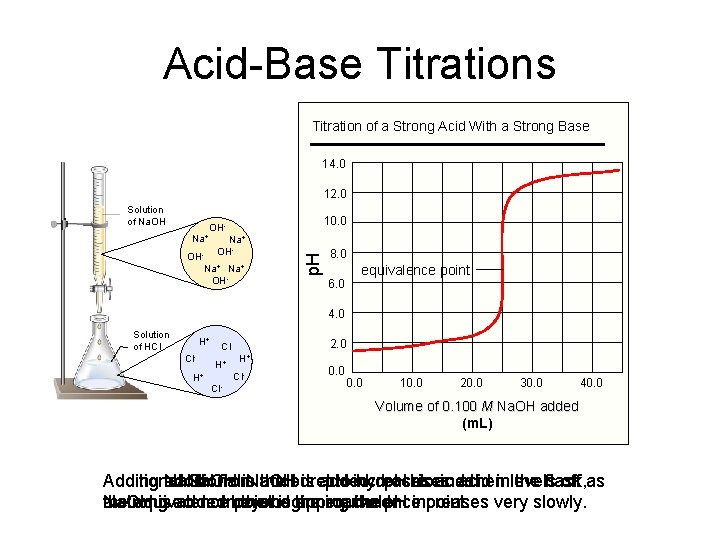
Acid-Base Titrations Titration of a Strong Acid With a Strong Base 14. 0 12. 0 Solution of Na. OH Na+ 10. 0 OHNa+ OH- OH Na+ OH- p. H - 8. 0 6. 0 equivalence point 4. 0 Solution of HCl H+ Cl. H+ 2. 0 Cl H+ H+ Cl- 0. 0 10. 0 20. 0 30. 0 40. 0 Volume of 0. 100 M Na. OH added (m. L) Additional Adding additional Na. OH from is. Na. OH added. the buret is added. p. H to increases hydrochloric p. H rises and as acid theninlevels the flask, off as the Na. OH a strong equivalence is acid. added. Inbeyond point the beginning is the approached. equivalence the p. H increases point. very slowly.

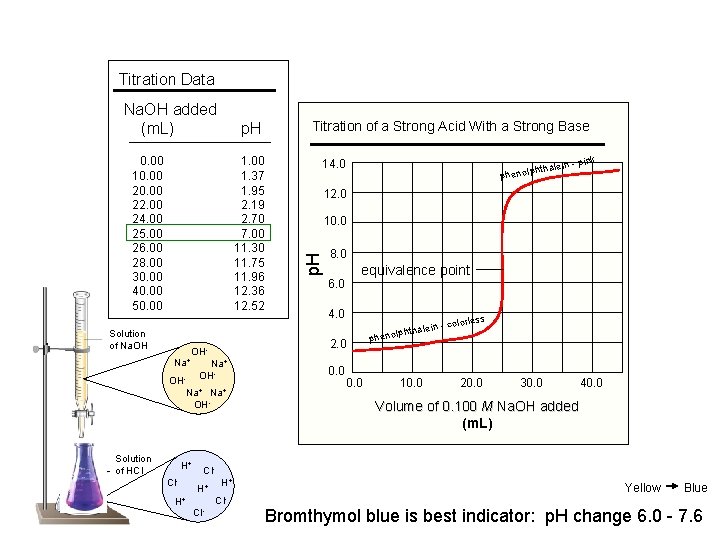
Titration Data p. H 0. 00 10. 00 22. 00 24. 00 25. 00 26. 00 28. 00 30. 00 40. 00 50. 00 1. 37 1. 95 2. 19 2. 70 7. 00 11. 30 11. 75 11. 96 12. 36 12. 52 Solution of Na. OH Na+ H+ Cl- 25 m. L H+ 14. 0 htha phenolp Na+ OH- ink lein - p 12. 0 10. 0 8. 0 6. 0 equivalence point 4. 0 s olorles -c hthalein lp o n e ph 2. 0 OH- OHNa+ OH- Solution of HCl Titration of a Strong Acid With a Strong Base p. H Na. OH added (m. L) 0. 0 10. 0 20. 0 30. 0 40. 0 Volume of 0. 100 M Na. OH added (m. L) Cl. H+ H+ Cl- Yellow Blue Bromthymol blue is best indicator: p. H change 6. 0 - 7. 6

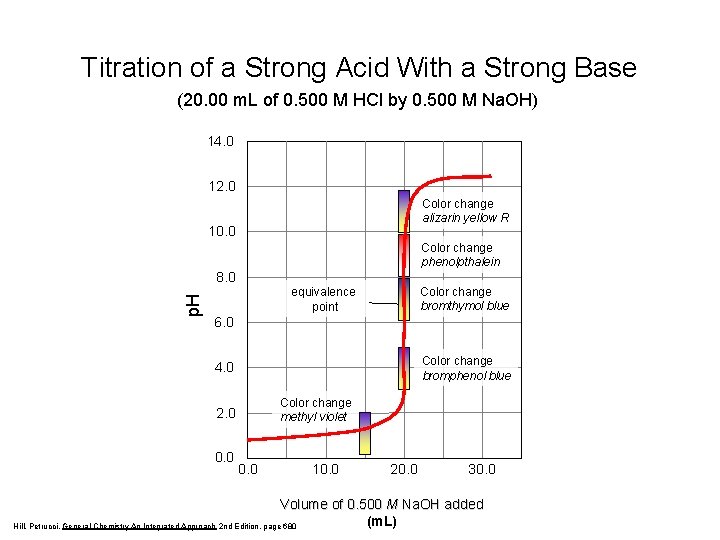
Titration of a Strong Acid With a Strong Base (20. 00 m. L of 0. 500 M HCl by 0. 500 M Na. OH) 14. 0 12. 0 Color change alizarin yellow R 10. 0 Color change phenolpthalein p. H 8. 0 Color change bromthymol blue equivalence point 6. 0 Color change bromphenol blue 4. 0 Color change methyl violet 2. 0 0. 0 10. 0 20. 0 30. 0 Volume of 0. 500 M Na. OH added (m. L) Hill, Petrucci, General Chemistry An Integrated Approach 2 nd Edition, page 680

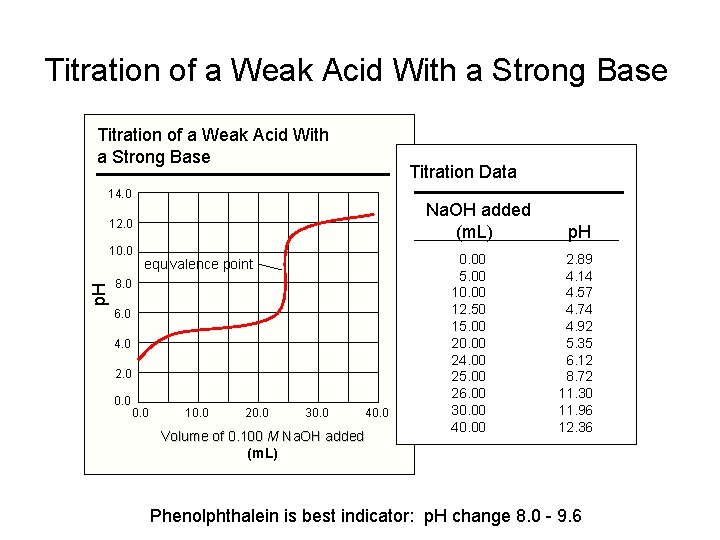
Titration of a Weak Acid With a Strong Base Titration Data 14. 0 Na. OH added (m. L) 12. 0 p. H 10. 0 equivalence point 8. 0 6. 0 4. 0 2. 0 0. 0 10. 0 20. 0 30. 0 Volume of 0. 100 M Na. OH added (m. L) 40. 00 5. 00 10. 00 12. 50 15. 00 20. 00 24. 00 25. 00 26. 00 30. 00 40. 00 p. H 2. 89 4. 14 4. 57 4. 74 4. 92 5. 35 6. 12 8. 72 11. 30 11. 96 12. 36 Phenolphthalein is best indicator: p. H change 8. 0 - 9. 6

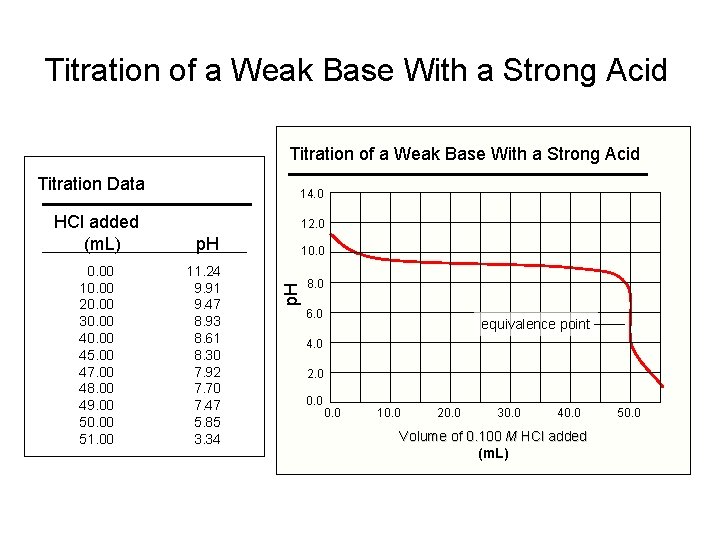
Titration of a Weak Base With a Strong Acid Titration Data 14. 0 HCl added (m. L) p. H 0. 00 10. 00 20. 00 30. 00 45. 00 47. 00 48. 00 49. 00 50. 00 51. 00 11. 24 9. 91 9. 47 8. 93 8. 61 8. 30 7. 92 7. 70 7. 47 5. 85 3. 34 12. 0 p. H 10. 0 8. 0 6. 0 equivalence point 4. 0 2. 0 0. 0 10. 0 20. 0 30. 0 40. 0 Volume of 0. 100 M HCl added (m. L) 50. 0

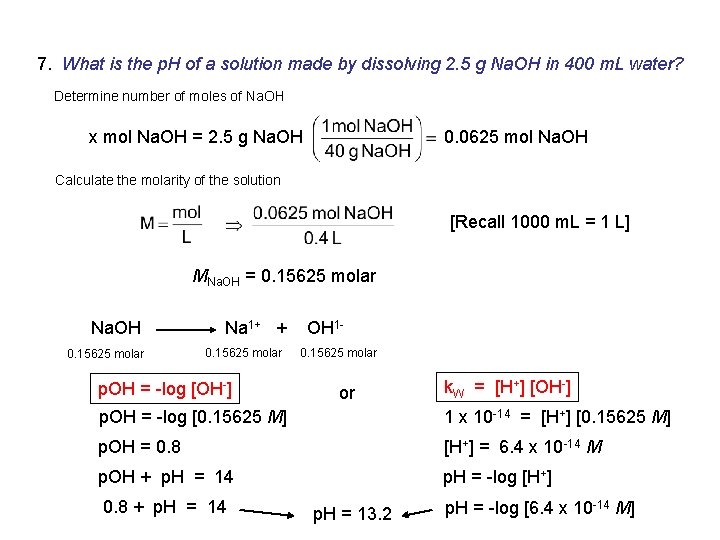
7. What is the p. H of a solution made by dissolving 2. 5 g Na. OH in 400 m. L water? Determine number of moles of Na. OH x mol Na. OH = 2. 5 g Na. OH 0. 0625 mol Na. OH Calculate the molarity of the solution [Recall 1000 m. L = 1 L] MNa. OH = 0. 15625 molar Na. OH 0. 15625 molar Na 1+ + 0. 15625 molar p. OH = -log [OH-] OH 10. 15625 molar or k. W = [H+] [OH-] p. OH = -log [0. 15625 M] 1 x 10 -14 = [H+] [0. 15625 M] p. OH = 0. 8 [H+] = 6. 4 x 10 -14 M p. OH + p. H = 14 p. H = -log [H+] 0. 8 + p. H = 14 p. H = 13. 2 p. H = -log [6. 4 x 10 -14 M]

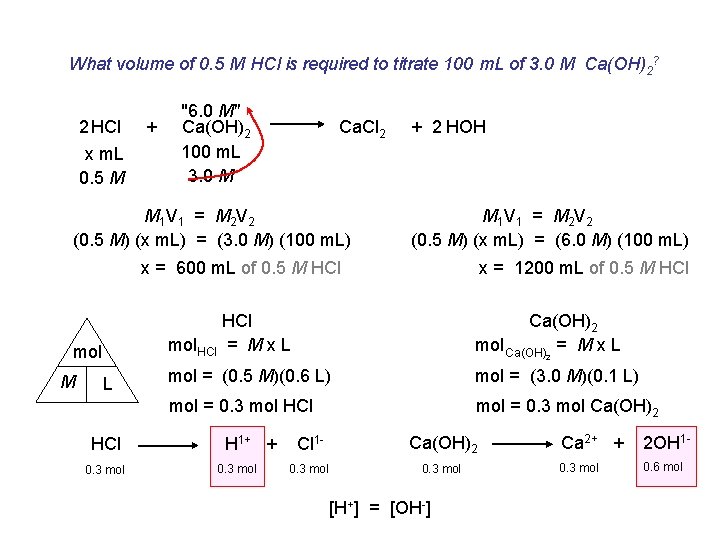
What volume of 0. 5 M HCl is required to titrate 100 m. L of 3. 0 M Ca(OH)2? 2 HCl x m. L 0. 5 M + "6. 0 M" Ca(OH)2 100 m. L 3. 0 M Ca. Cl 2 M 1 V 1 = M 2 V 2 (0. 5 M) (x m. L) = (3. 0 M) (100 m. L) + 2 HOH M 1 V 1 = M 2 V 2 (0. 5 M) (x m. L) = (6. 0 M) (100 m. L) x = 600 m. L of 0. 5 M HCl mol M L HCl 0. 3 mol x = 1200 m. L of 0. 5 M HCl mol. HCl = M x L Ca(OH)2 mol Ca(OH) = M x L mol = (0. 5 M)(0. 6 L) mol = (3. 0 M)(0. 1 L) mol = 0. 3 mol HCl mol = 0. 3 mol Ca(OH)2 H 1+ + 0. 3 mol 2 Cl 1 - Ca(OH)2 0. 3 mol [H+] = [OH-] Ca 2+ + 2 OH 1 - 0. 3 mol 0. 6 mol

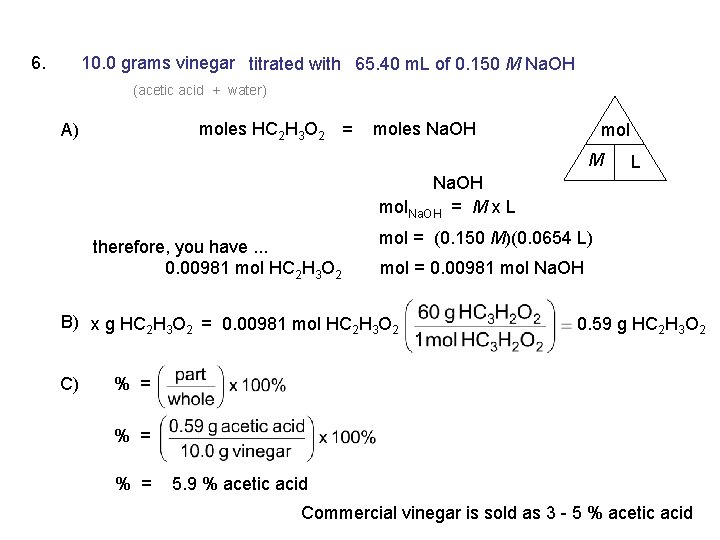
6. 10. 0 grams vinegar titrated with 65. 40 m. L of 0. 150 M Na. OH (acetic acid + water) moles HC 2 H 3 O 2 = A) moles Na. OH mol M L Na. OH mol. Na. OH = M x L therefore, you have. . . 0. 00981 mol HC 2 H 3 O 2 mol = (0. 150 M)(0. 0654 L) mol = 0. 00981 mol Na. OH B) x g HC 2 H 3 O 2 = 0. 00981 mol HC 2 H 3 O 2 C) 0. 59 g HC 2 H 3 O 2 % = % = 5. 9 % acetic acid Commercial vinegar is sold as 3 - 5 % acetic acid

Carboxylic Acid HC 2 H 3 O 2 = acetic acid : : H O H C C 1 O H H H+ CH 3 COOH R - COOH carboxylic acid C 2 H 4 O 2

O H C O H H C H H

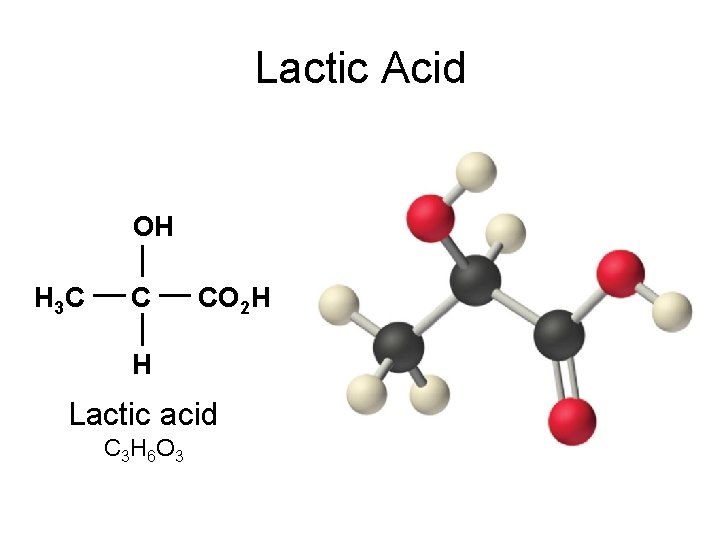
Lactic Acid OH H 3 C C CO 2 H H Lactic acid C 3 H 6 O 3

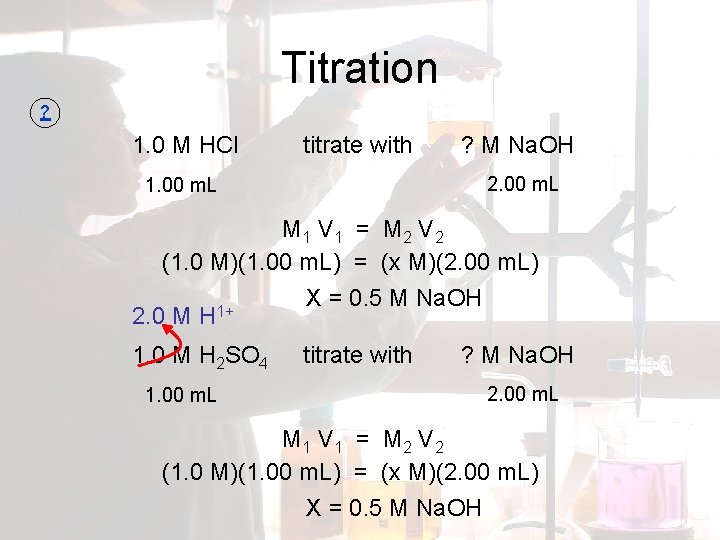
Titration ? 1. 0 M HCl titrate with ? M Na. OH 2. 00 m. L 1. 00 m. L M 1 V 1 = M 2 V 2 (1. 0 M)(1. 00 m. L) = (x M)(2. 00 m. L) X = 0. 5 M Na. OH 1+ 2. 0 M H 1. 0 M H 2 SO 4 1. 00 m. L titrate with ? M Na. OH 2. 00 m. L M 1 V 1 = M 2 V 2 (1. 0 M)(1. 00 m. L) = (x M)(2. 00 m. L) X = 0. 5 M Na. OH