Titration Part 1 Making a primary standard solution
- Slides: 3

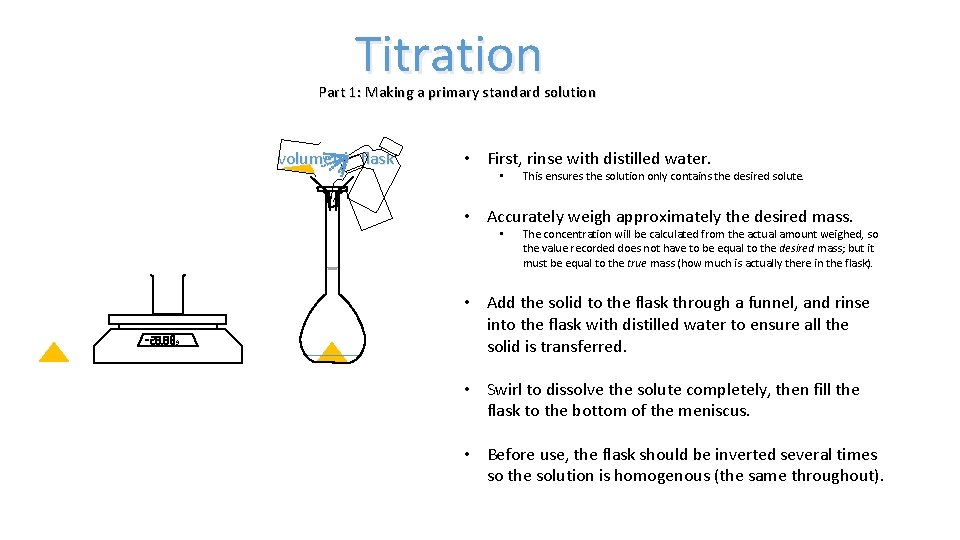
Titration Part 1: Making a primary standard solution volumetric flask • First, rinse with distilled water. • This ensures the solution only contains the desired solute. • Accurately weigh approximately the desired mass. • The concentration will be calculated from the actual amount weighed, so the value recorded does not have to be equal to the desired mass; but it must be equal to the true mass (how much is actually there in the flask). • Add the solid to the flask through a funnel, and rinse into the flask with distilled water to ensure all the solid is transferred. • Swirl to dissolve the solute completely, then fill the flask to the bottom of the meniscus. • Before use, the flask should be inverted several times so the solution is homogenous (the same throughout).

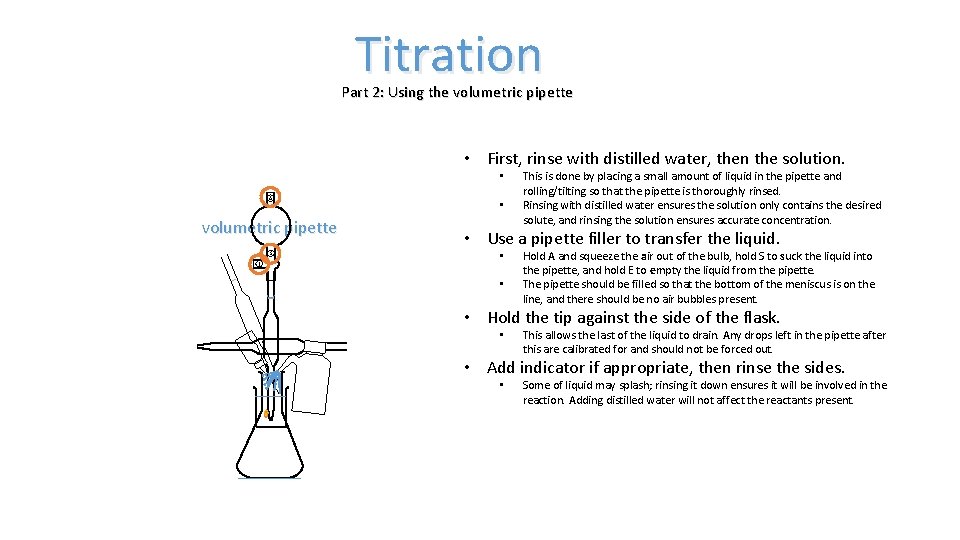
Titration Part 2: Using the volumetric pipette • First, rinse with distilled water, then the solution. • A volumetric pipette S E • This is done by placing a small amount of liquid in the pipette and rolling/tilting so that the pipette is thoroughly rinsed. Rinsing with distilled water ensures the solution only contains the desired solute, and rinsing the solution ensures accurate concentration. • Use a pipette filler to transfer the liquid. • • Hold A and squeeze the air out of the bulb, hold S to suck the liquid into the pipette, and hold E to empty the liquid from the pipette. The pipette should be filled so that the bottom of the meniscus is on the line, and there should be no air bubbles present. • Hold the tip against the side of the flask. • This allows the last of the liquid to drain. Any drops left in the pipette after this are calibrated for and should not be forced out. • Add indicator if appropriate, then rinse the sides. • Some of liquid may splash; rinsing it down ensures it will be involved in the reaction. Adding distilled water will not affect the reactants present.

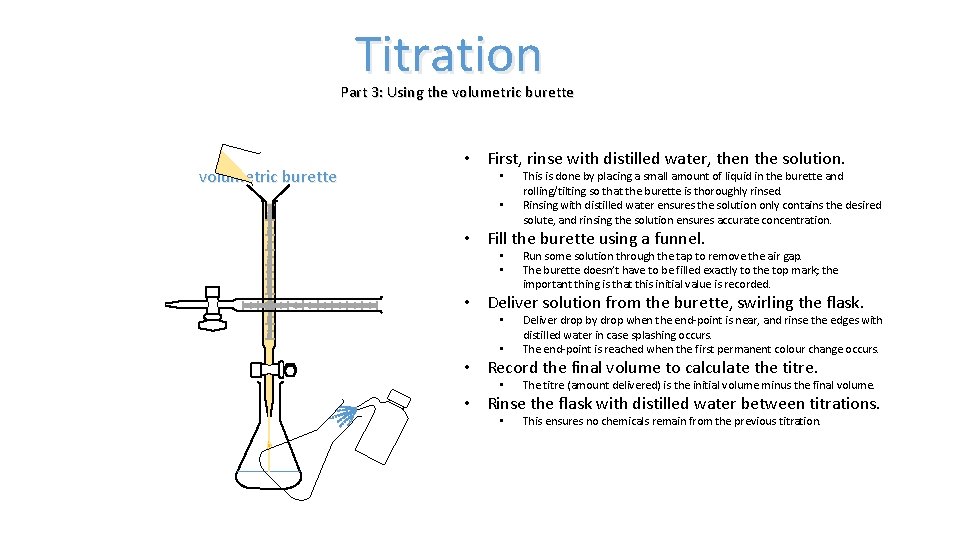
Titration Part 3: Using the volumetric burette • First, rinse with distilled water, then the solution. • • This is done by placing a small amount of liquid in the burette and rolling/tilting so that the burette is thoroughly rinsed. Rinsing with distilled water ensures the solution only contains the desired solute, and rinsing the solution ensures accurate concentration. • Fill the burette using a funnel. • • Run some solution through the tap to remove the air gap. The burette doesn’t have to be filled exactly to the top mark; the important thing is that this initial value is recorded. • Deliver solution from the burette, swirling the flask. • • Deliver drop by drop when the end-point is near, and rinse the edges with distilled water in case splashing occurs. The end-point is reached when the first permanent colour change occurs. • Record the final volume to calculate the titre. • The titre (amount delivered) is the initial volume minus the final volume. • Rinse the flask with distilled water between titrations. • This ensures no chemicals remain from the previous titration.