Titration Acids Bases Acid Dissociation Constant Ka The

Titration Acids & Bases
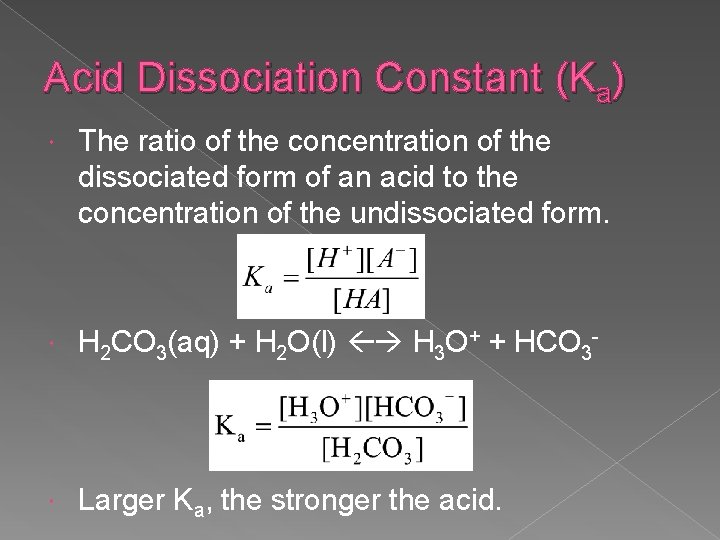
Acid Dissociation Constant (Ka) The ratio of the concentration of the dissociated form of an acid to the concentration of the undissociated form. H 2 CO 3(aq) + H 2 O(l) H 3 O+ + HCO 3 - Larger Ka, the stronger the acid.
Neutralization Chemical reaction between an acid and a base. Products are a salt (ionic compound) and water.
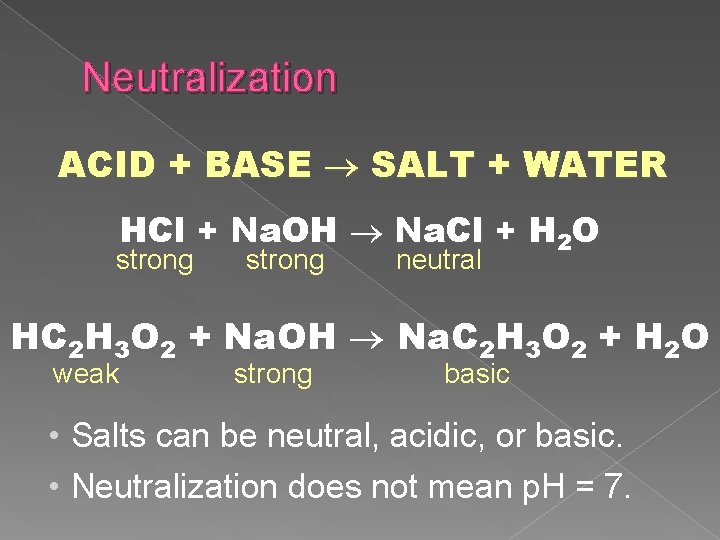
Neutralization ACID + BASE SALT + WATER HCl + Na. OH Na. Cl + H 2 O strong neutral HC 2 H 3 O 2 + Na. OH Na. C 2 H 3 O 2 + H 2 O weak strong basic • Salts can be neutral, acidic, or basic. • Neutralization does not mean p. H = 7.

Titration standard solution › Analytical method in which a standard solution is used to determine the concentration of an unknown solution
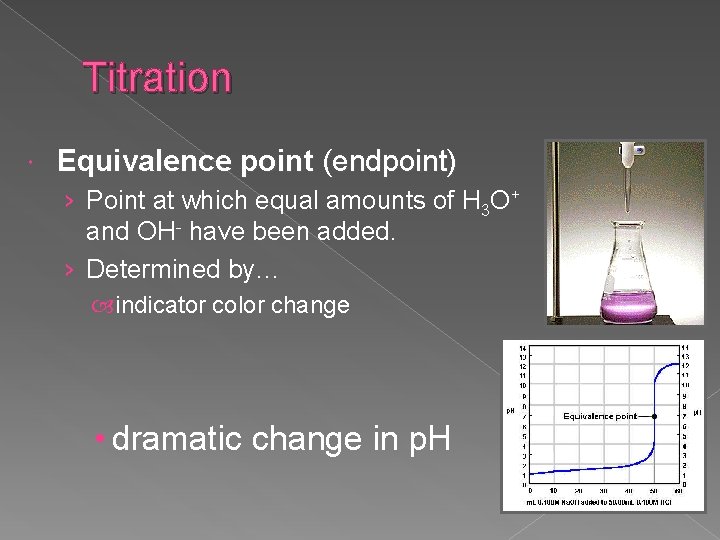
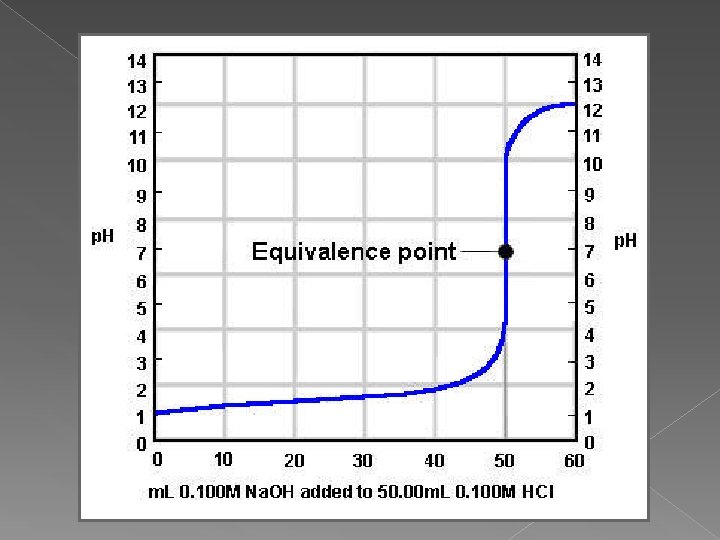
Titration Equivalence point (endpoint) › Point at which equal amounts of H 3 O+ and OH- have been added. › Determined by… indicator color change • dramatic change in p. H
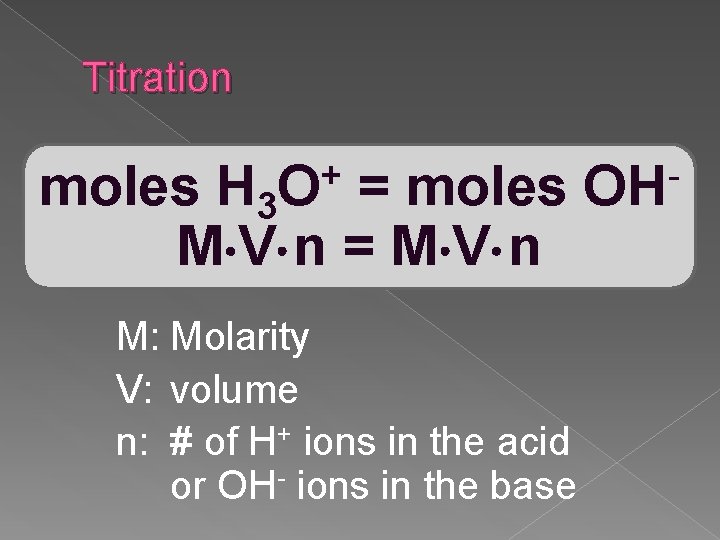
Titration + O moles H 3 = moles M V n = M V n M: Molarity V: volume n: # of H+ ions in the acid or OH- ions in the base OH
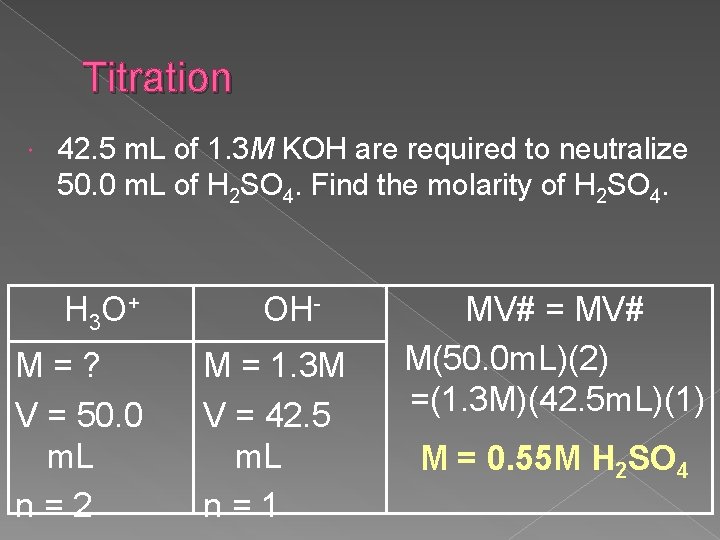
Titration 42. 5 m. L of 1. 3 M KOH are required to neutralize 50. 0 m. L of H 2 SO 4. Find the molarity of H 2 SO 4. H 3 O + M=? V = 50. 0 m. L n=2 OHM = 1. 3 M V = 42. 5 m. L n=1 MV# = MV# M(50. 0 m. L)(2) =(1. 3 M)(42. 5 m. L)(1) M = 0. 55 M H 2 SO 4

Buffer A solution of a weak acid and a weak base that resists large p. H changes when acid or base is added. Buffer capacity: amount of acid or base that can be added without changing the p. H › Buffered Aspirin › Bufferin
- Slides: 10