TIPS FOR TAKING THE PAIN OUT OF PAIN























- Slides: 58

TIPS FOR TAKING THE PAIN OUT OF PAIN MANAGEMENT IN THE CKD PATIENT IN THE ERA OF THE OPIOID EPIDEMIC Tracy Anderson-Haag, Pharm. D. , BCPS Clinical Pharmacy Specialist Hennepin Healthcare & Clinical Assistant Professor University of Minnesota, College of Pharmacy Minneapolis, MN

OBJECTIVES § Identify common pain syndromes and their management in CKD § Introduce unique pharmacokinetic and pharmacodynamic alterations in CKD that affect efficacy and safety of opioid and nonopioid analgesics § Explore appropriate use of alternative and/or adjuvant agents to enhance efficacy and safety in the management of common pain syndromes in the CKD patient § Outline the scope of the opioid crisis in the US and current opioid usage patterns in patients with CKD and kidney transplants § Investigate safer opioid prescribing practices being implemented in the US

PAIN PRESENTATION ACUTE PAIN • “Complex, unpleasant experience with emotional and cognitive, as well as sensory, features that occur in response to tissue trauma” • Response to tissue trauma • Activation of nociceptors and/or central neurons • CNS active medications (opioids, anticonvulsants, antidepressants) alleviate pain by interacting with opioid receptors and neurochemicals CHRONIC PAIN • Response to prolonged tissue injury; persistent activation of nociceptors or somatosensory system • Non-neuropathic • Intense, dull, deep pain • Neuropathic (somatosensory system) • Intense hot, cold, sensitive, itchy, surface pain

PAIN IN GENERAL US ADULT POPULATION § 126. 1 million reported pain within 3 months (~ 50% of population) § 25. 3 million reported chronic pain daily § 23. 4 million reported pain as severe § Results: > 200 MILLION opioid prescriptions dispensed by retail pharmacies in 2012 Pham PC, et al. 2017 Update on Pain Management in Patients with CKD. Clinical Kidney Journal. 2017, 10(5): 688 -97.

PAIN IN CHRONIC KIDNEY DISEASE § Prevalence • 40 -60% on renal replacement therapy • 60 -70% for pre-ESKD • Up to 100% for hospitalized CKD patients § Majority is musculoskeletal • Joint/arthritis > lower back pain Pham PC, et al. 2017 Update on Pain Management in Patients with CKD. Clinical Kidney Journal. 2017, 10(5): 688 -97.

ETIOLOGY OF PAIN IN CHRONIC KIDNEY DISEASE § Ischemic limbs § Joint pain/arthritis § Neuropathy § Headaches § Polycystic kidneys § Nephrolithiasis § Secondary Hyperparathyroidism § Calciphylaxis § Bone pain § Cramping § Access • Needles • Steal syndrome

PAIN MANAGEMENT TOOLBOX dol a m Tra s id Opio n- No APA Dr Topic ug als AI NS tin DS en p a b a G P

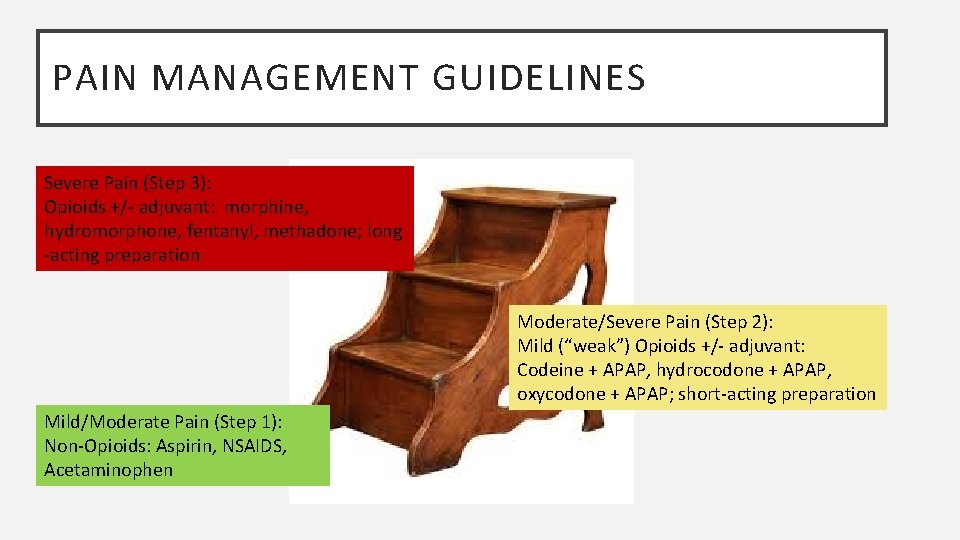
PAIN MANAGEMENT GUIDELINES Severe Pain (Step 3): Opioids +/- adjuvant: morphine, hydromorphone, fentanyl, methadone; long -acting preparation Moderate/Severe Pain (Step 2): Mild (“weak”) Opioids +/- adjuvant: Codeine + APAP, hydrocodone + APAP, oxycodone + APAP; short-acting preparation Mild/Moderate Pain (Step 1): Non-Opioids: Aspirin, NSAIDS, Acetaminophen

TOOLS: PAIN MEDICATIONS NON-OPIOIDS § APAP § NSAIDs/aspirin § Tricyclic antidepressants § Muscle relaxants § Antidepressants (SSRI, SNRI) § Antihypertensives (Beta-blockers, CCB) § Anticonvulsants § Corticosteroids SELECT OPIOIDS • • Tramadol Hydromorphone Fentanyl Oxycodone Hydrocodone Codeine Meperidine Methadone

TOOLS: NON-DRUG THERAPY OTHER PROVIDERS INTERVENTIONS § Physical Therapist • Heat/Ice § Massage Therapist • Stretching § Acupuncturist • Biofeedback/relaxation therapy § Chiropractor • Yoga • Daily activity • Transcutaneous Electrical Nerve Stimulation (TENS)

PAIN MANAGEMENT GUIDELINES

The longer you play, the harder it gets!!

OPIOID EPIDEMIC: BAD MEDICINE § In 2016, opioids killed this many people in the US A) 5700 people B) 12, 000 people C) 36, 000 people D) 42, 000 people E) 50, 000 people

OPIOID EPIDEMIC: BAD MEDICINE § In 2016, opioids killed this many people in the US A) 5700 people B) 12, 000 people C) 36, 000 people D) 42, 000 people E) 50, 000 people

OPIOID EPIDEMIC: BAD MEDICINE § In 2016, opioids killed this many people in the US A) 5700 people B) 12, 000 people C) 36, 000 people D) 42, 000 people E) 50, 000 people That’s 116 people per 40% involve a prescription DAY opioid Rx

OPIOIDS IN DIALYSIS § Maintenance dialysis patients with Medicare A, B and D § > 60% of dialysis patients had ≥ 1 opioid prescription each year § 20% of dialysis patients had a chronic opioid prescription (≥ 90 day supply) each year § Patients with opioid prescriptions had increased mortality, dialysis discontinuation and hospitalization § Higher doses correlated with higher mortality risk • > 25% of patients with Morphine Milligram Equivalent “MME” > 50 per day Kimmel P, et al. Opioid Prescription, Morbidity, and Mortality in US Dialysis Patients. JASN. 2017; 28: 3658 -3670.

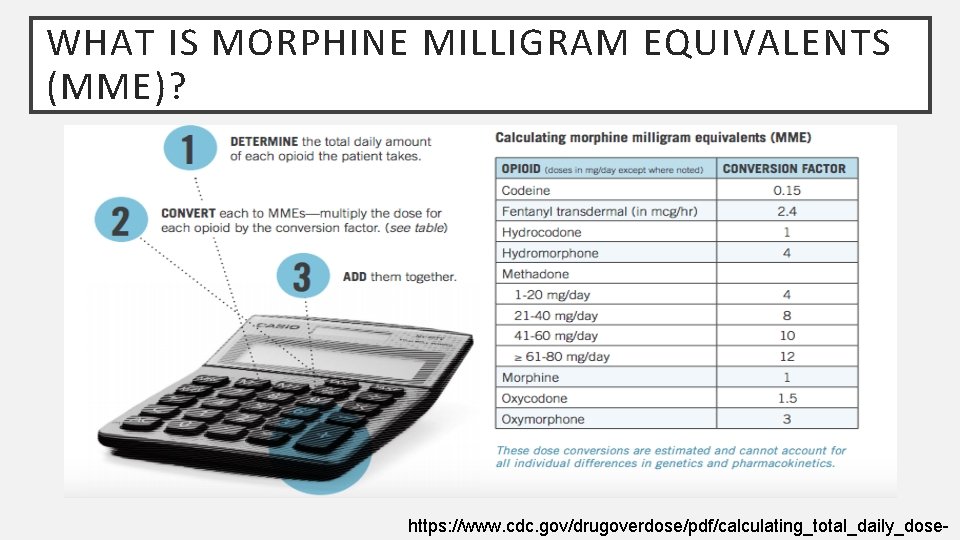
WHAT IS MORPHINE MILLIGRAM EQUIVALENTS (MME)? https: //www. cdc. gov/drugoverdose/pdf/calculating_total_daily_dose-

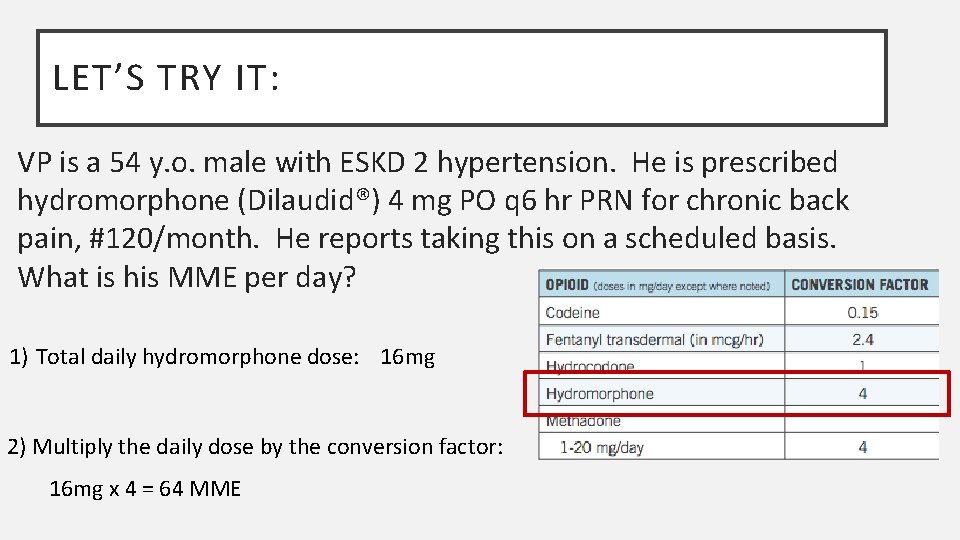
LET’S TRY IT: VP is a 54 y. o. male with ESKD 2 hypertension. He is prescribed hydromorphone (Dilaudid®) 4 mg PO q 6 hr PRN for chronic back pain, #120/month. He reports taking this on a scheduled basis. What is his MME per day? 1) Total daily hydromorphone dose: 16 mg 2) Multiply the daily dose by the conversion factor: 16 mg x 4 = 64 MME

OPIOIDS IN DIALYSIS § Koncicki et al. reported increased risk of respiratory depression and sedation with MME > 50 per day § Ishida et al. • N=140, 899 Medicare-covered adults, in-center HD (2011) • 64% had opioid prescription, 23% at least 1 “high-dose” prescription (>60 MME) Ø Hydrocodone 43%, oxycodone (22%), tramadol (15%), codeine (7%), hydromorphone (3%), fentanyl (3%), morphine (2%), methadone (1%) • 17% of patients had episode of altered MS, fall or fracture • HR 1. 28 -1. 65 for any opioid use vs none for all outcomes Koncicki HM, et al. Seminars in Dialysis. 2015; 28: 384 -91. Ishida JH, et al. CJASN. 2018; 13: 746 -753.

OPIOIDS IN KIDNEY TRANSPLANT § N=36, 486 (2010 prevalent cohort) § 14. 6% had long term opioid prescription § Increased risk of mortality and graft loss compared to no long term opioid prescription, or only short term opioid § Highest risk with highest dose; group of ≥ 90 MME per day (HR 1. 61 for death, 1. 33 for graft loss) Abbott KC, et al. Transplantation. 2018; 102(6): 994 -1004.

“Tylenol doesn’t work for me. ” “The only thing that works is Dilaudid. ” --Chronic kidney disease patient

OPIOIDS VS NON-OPIOIDS FOR CHRONIC PAIN Krebs EE, et al. JAMA. 2018; 319(9): 872 -882

THE SPACE TRIAL § Objective: To compare opioid vs non-opioid medications over 12 months on pain-related function, pain intensity and ADR § Pragmatic, 12 -month, randomized; masked assessment • N=240 • Primary care patients with chronic back pain or hip or knee osteoarthritis pain; at least moderate severity on analgesics Krebs EE, et al. JAMA. 2018; 319(9): 872 -882

THE SPACE TRIAL § Treat to target strategy in both arms • Opioid (titrated to max dose of “ 100 MME”) Ø Step 1: morphine IR, oxycodone or hydrocodone/APAP Ø Step 2: morphine ER, oxycodone ER, Ø Step 3: fentanyl patch • Non-opioid Ø Step 1: acetaminophen or NSAID Ø Step 2: nortriptyline, amitriptyline, gabapentin, topical analgesics (capsaicin, lidocaine) Ø Step 3: pregabalin, duloxetine, tramadol Krebs EE, et al. JAMA. 2018; 319(9): 872 -882

THE SPACE TRIAL: RESULTS § Mean age 58. 3 years, 13% women § 65% back pain, 35% hip or knee osteoarthritis § Opioid group 21% smokers, non-opioid group 11% smokers § No significant difference in pain-related function over 12 months § Pain intensity was significantly better in the non-opioid group over 12 months (P=0. 05) § HR-Qo. L did not differ between groups § Opioid group had more side effects over 12 months than non-opioid group Krebs EE, et al. JAMA. 2018; 319(9): 872 -882

THE SPACE TRIAL: CONCLUSION § “Treatment with opioids was not superior to treatment with non-opioid medications for improving pain-related function over 12 months” Krebs EE, et al. JAMA. 2018; 319(9): 872 -882

“Tylenol can hurt my liver. I’m not taking that. I already have kidney disease. ” --Chronic kidney disease patient

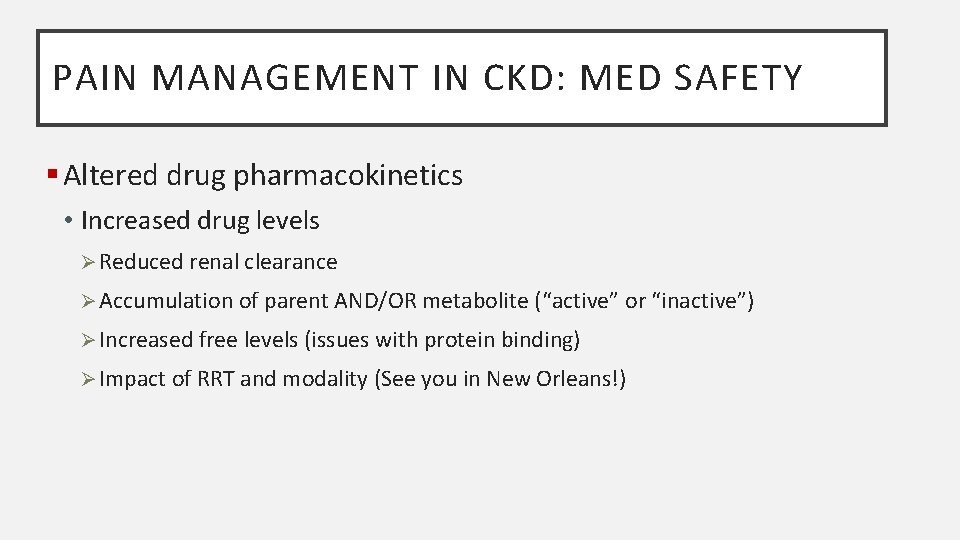
PAIN MANAGEMENT IN CKD: MED SAFETY § Altered drug pharmacokinetics • Increased drug levels Ø Reduced renal clearance Ø Accumulation of parent AND/OR metabolite (“active” or “inactive”) Ø Increased free levels (issues with protein binding) Ø Impact of RRT and modality (See you in New Orleans!)

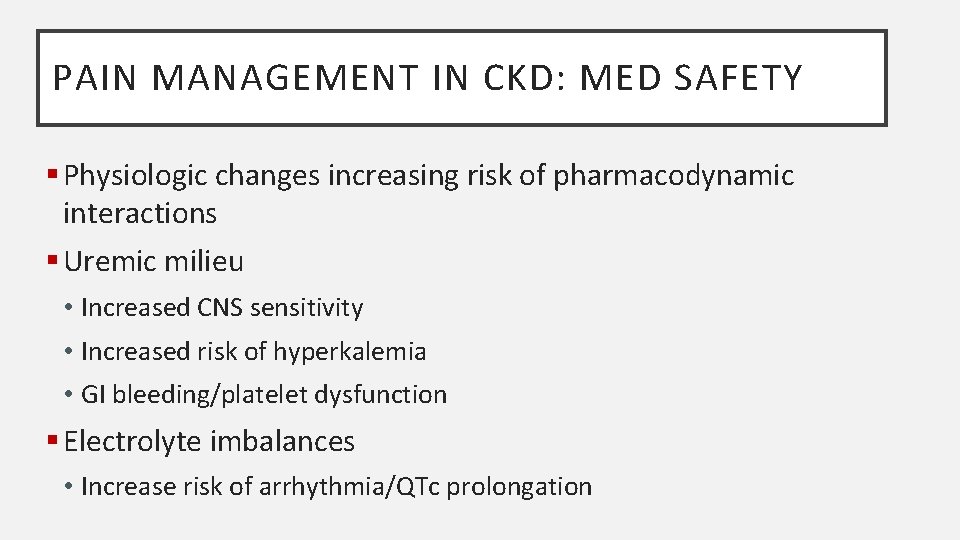
PAIN MANAGEMENT IN CKD: MED SAFETY § Physiologic changes increasing risk of pharmacodynamic interactions § Uremic milieu • Increased CNS sensitivity • Increased risk of hyperkalemia • GI bleeding/platelet dysfunction § Electrolyte imbalances • Increase risk of arrhythmia/QTc prolongation

NON-OPIOID MEDICATIONS * *Summary of references at end of handout

TOPICAL AGENTS § Access cannulation • Lidocaine or prilocaine cream • Vapocoolant spray § Localized neuropathic or musculoskeletal pain • Lidocaine patches § Topical NSAIDs? • Diclofenac (Voltaren) Ø Caution warranted Ø Case reports of AKI

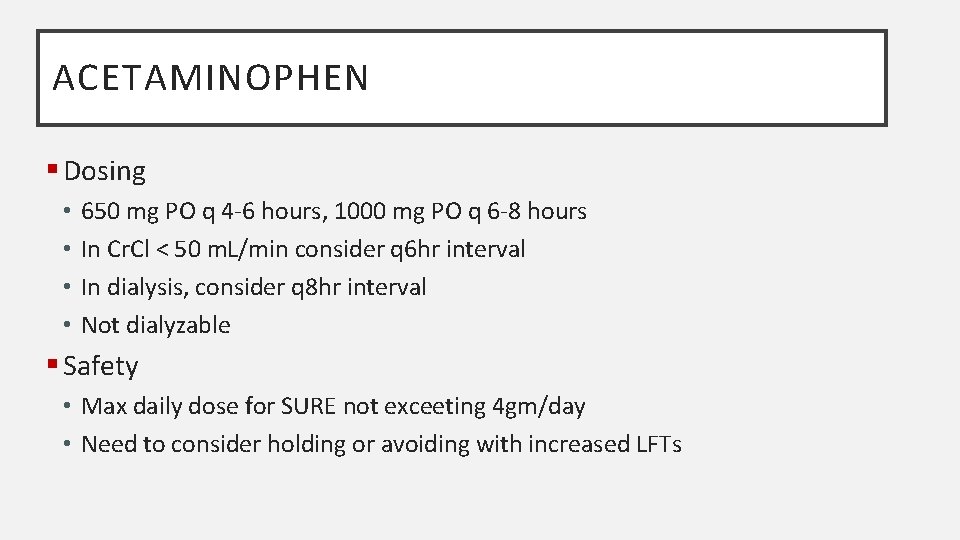
ACETAMINOPHEN § Dosing • • 650 mg PO q 4 -6 hours, 1000 mg PO q 6 -8 hours In Cr. Cl < 50 m. L/min consider q 6 hr interval In dialysis, consider q 8 hr interval Not dialyzable § Safety • Max daily dose for SURE not exceeting 4 gm/day • Need to consider holding or avoiding with increased LFTs

NON-STEROIDAL ANTI-INFLAMMATORY DRUGS § NSAIDs • Ibuprofen, naproxen, ketorolac, indomethacin, high-dose aspirin; COX -2 inhibitors (celecoxib) • Concerns with nephrotoxicity Ø Chronic interstitial fibrosis/interstitial nephritis Ø Hemodynamically-Induced • Platelet effects: increased risk of GI bleed • Worsening hypertension, volume retention, hyperkalemia

NSAID AKI RISK FACTORS § CKD § Hypoalbuminemia § Volume Depletion § Nephrotic Syndrome § CHF/CAD § Diabetes Mellitus § Liver failure with cirrhosis § Sepsis § Concomitant use of nephrotoxic medication(s) § High drug dose § Diuretic use What about when my patient is already on hemodialysis?

GABAPENTIN (NEURONTIN ® ) § Indicated for epilepsy and postherpetic neuralgia • Used for many other conditions (neuropathic pain syndromes, uremic pruritis, RLS) § Primarily removed by the kidneys § Usual dose • Often initiated at 100 -300 mg PO QOD to daily, titrated up to response & tolerance • Caution in kidney disease Ø Slow up-titration (not more than one increase per week) Ø Generally do not exceed 300 mg daily in dialysis crowd Ø Some is removed by dialysis; give after run/QHS

GABAPENTIN (NEURONTIN ® ) § Adverse Effects • Somnolence, dizziness, mental status changes, ataxia, respiratory depression § Drug Interactions • Concurrent sedating medications • ? Increased exposure when used concomitantly with opioids § Watch for more • Gomes T, et al. Gabapentin, opioids, and the risk of opioid-related death: A population-based, nested case-control study. PLOS Medicine. October 3, 2017 l 1 -13. https: //doi. org/10. 1371/journal. pmed. 1002396. • Opioids + gabapentin, increased risk of opioid-related death

PREGABALIN (LYRICA ® ) § Produces inhibitory modulation of neuronal excitability in the CNS; act on calcium channels § Schedule V controlled substance § Indicated • • Diabetic peripheral neuropathy Postherpetic neuralgia Adjunctive therapy for partial onset seizures Fibromyalgia § Primarily removed by the kidney

PREGABALIN (LYRICA ® ) § Usual dose • Normal kidney function: Starting doses 75 mg BID or 50 mg TID, can increase in 1 week if tolerating and need additional effect • Dose must be reduced for CKD (see table) • When discontinuing, taper over minimum of one week • Removal by hemodialysis Ø 50% removal in 4 hours Ø Give daily dose after dialysis Ø Supplemental dose?

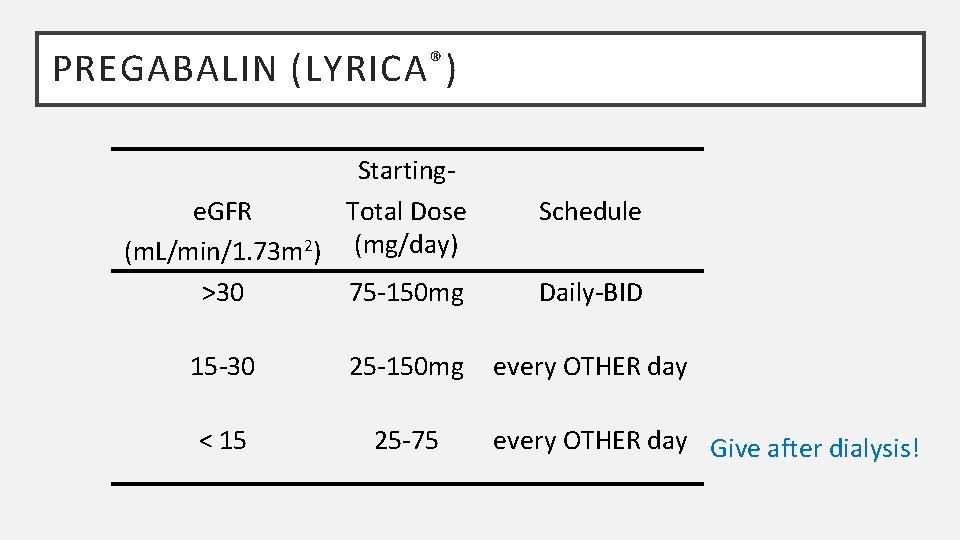
PREGABALIN (LYRICA ® ) Startinge. GFR Total Dose (m. L/min/1. 73 m 2) (mg/day) >30 75 -150 mg 15 -30 25 -150 mg < 15 25 -75 Schedule Daily-BID every OTHER day Give after dialysis!

PREGABALIN (LYRICA ® ) § Adverse Effects • Dizziness, somnolence, dry mouth, edema, weight gain, ataxia, confusion, abnormal thinking/attention span • Use caution with dose escalation in CKD, elderly § Drug Interactions • Sedating medications (narcotics, benzos, ETOH)

TRICYCLIC ANTIDEPRESSANTS (TCAS) § Effective for neuropathic pain and sleep § Safety concerns with anticholinergic effects, especially in older adults § Desipramine, nortriptyline with least anticholinergic effect

DULOXETINE (CYMBALTA ® ) § Indications • Major Depressive Disorder, Diabetic Peripheral Neuropathic Pain, Generalized Anxiety Disorder § Mechanism of Action: dual serotonin and norepinephrine reuptake inhibitor § Dosage • 60 mg po once daily • May try 30 mg dose first (especially elderly) • Not recommended in CKD patients with Cr. Cl < 30 m. L/min (100% increased exposure in dialysis patients) § Patient Instructions • Do not abruptly stop medication (withdrawal)

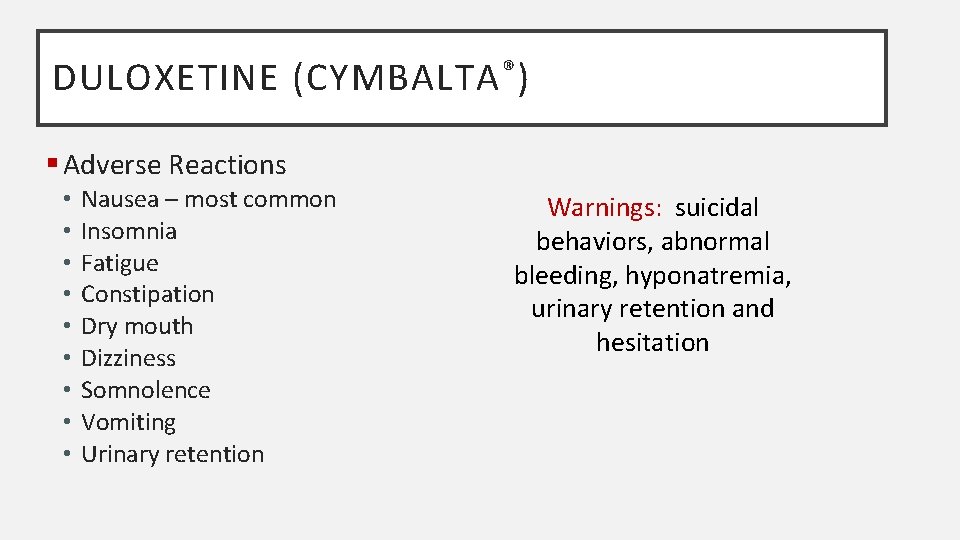
DULOXETINE (CYMBALTA ® ) § Adverse Reactions • • • Nausea – most common Insomnia Fatigue Constipation Dry mouth Dizziness Somnolence Vomiting Urinary retention Warnings: suicidal behaviors, abnormal bleeding, hyponatremia, urinary retention and hesitation

OPIOID MEDICATIONS * *Summary of references at end of handout

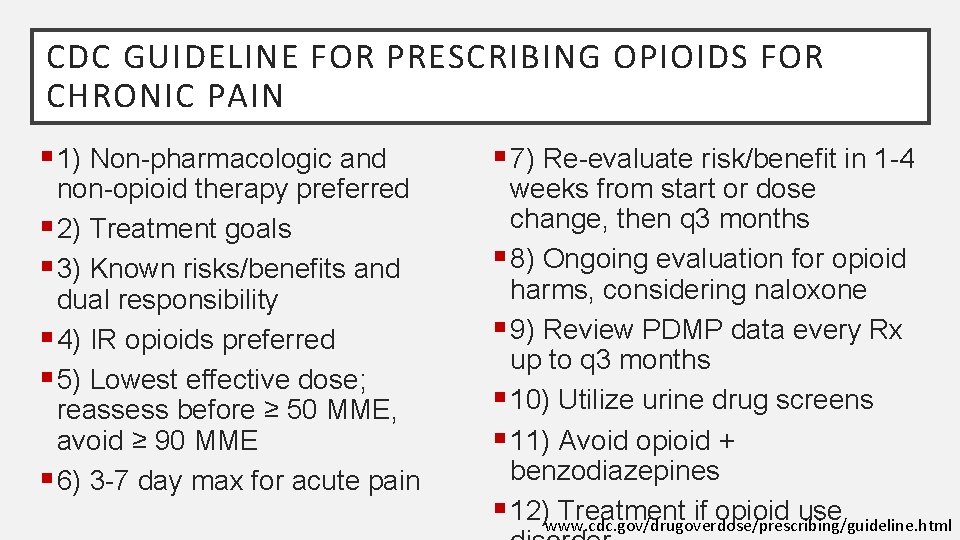
CDC GUIDELINE FOR PRESCRIBING OPIOIDS FOR CHRONIC PAIN § 1) Non-pharmacologic and non-opioid therapy preferred § 2) Treatment goals § 3) Known risks/benefits and dual responsibility § 4) IR opioids preferred § 5) Lowest effective dose; reassess before ≥ 50 MME, avoid ≥ 90 MME § 6) 3 -7 day max for acute pain § 7) Re-evaluate risk/benefit in 1 -4 weeks from start or dose change, then q 3 months § 8) Ongoing evaluation for opioid harms, considering naloxone § 9) Review PDMP data every Rx up to q 3 months § 10) Utilize urine drug screens § 11) Avoid opioid + benzodiazepines § 12)www. cdc. gov/drugoverdose/prescribing/guideline. html Treatment if opioid use

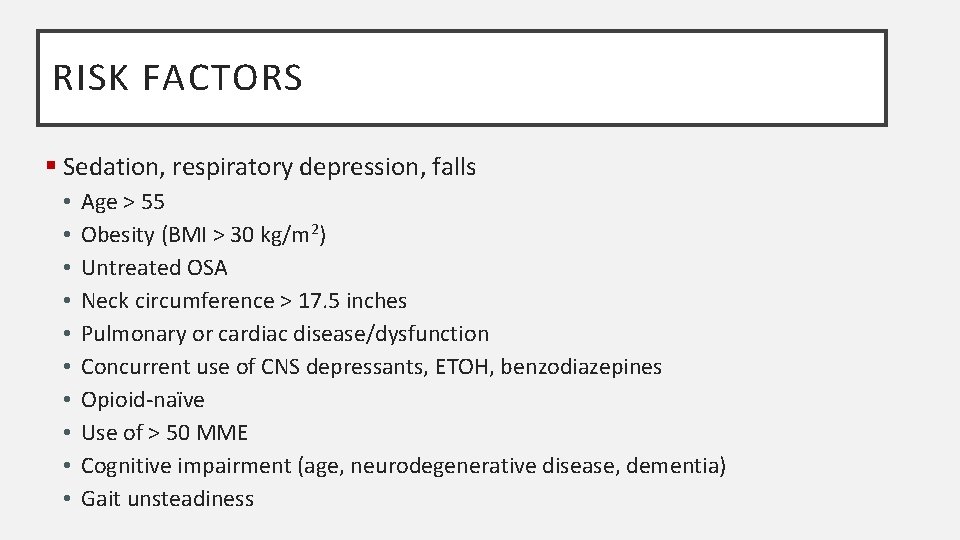
RISK FACTORS § Sedation, respiratory depression, falls • • • Age > 55 Obesity (BMI > 30 kg/m 2) Untreated OSA Neck circumference > 17. 5 inches Pulmonary or cardiac disease/dysfunction Concurrent use of CNS depressants, ETOH, benzodiazepines Opioid-naïve Use of > 50 MME Cognitive impairment (age, neurodegenerative disease, dementia) Gait unsteadiness

OPIOID MISUSE § Incidence in CKD unknown § Opioid Risk Tool • Administer to patients on initial visit before initiation of opioid therapy • https: //www. drugabuse. gov/sites/default/files/Opioid. Risk. Tool

OPIOID MEDICATIONS § Tramadol § Weak Opioids • Often in combination with acetaminophen 325 mg (T#3, Percocet, Norco) • Hydrocodone, oxycodone, codeine § Strong Opioids • Morphine, hydromorphone, fentanyl, meperidine, methadone, oxycodone

AVOIDANCE GENERALLY RECOMMENDED § Meperidine • Accumulation of of normeperidine; seizures, AMS, myoclonus § Morphine • Active metabolite, morphine-6 -glucuronide accumulation; CNS depression, respiratory depression, myoclonus, death § Codeine • Reduced clearance; N/V, hypotension, CNS depression, respiratory arrest § Hydrocodone • Limited data; reduced clearance of parent/metabolite § Methadone • Refer to a pain specialist

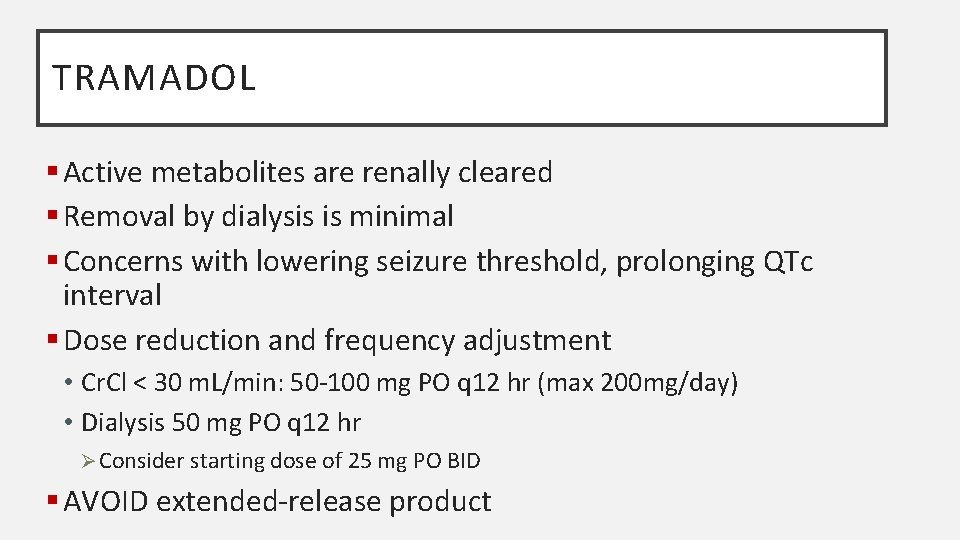
TRAMADOL § Active metabolites are renally cleared § Removal by dialysis is minimal § Concerns with lowering seizure threshold, prolonging QTc interval § Dose reduction and frequency adjustment • Cr. Cl < 30 m. L/min: 50 -100 mg PO q 12 hr (max 200 mg/day) • Dialysis 50 mg PO q 12 hr Ø Consider starting dose of 25 mg PO BID § AVOID extended-release product

OXYCODONE § Short-acting; caution necessary § Metabolized to noroxycodone and oxymorphone (active); half-lives increased § Peak plasma levels increased for oxycodone (50%) and noroxycodone (20%) in patients with Cr. Cl < 60 ml/min § AUC of oxycodone, noroxycodone, and oxymorphone increased 60, 50, and 40%, respectively § Up to 19% free oxycodone found in urine § Reported increase in sedation § AVOID Oxycontin®

HYDROMORPHONE § Preferred short-acting opioid in advanced CKD § Less neuroexcitatory effects (LESS not NONE) • Neuroexcitation and seizures have been reported with high doses § Hepatic and renal elimination • Half-life doubled in CKD not on dialysis • Accumulation has been reported • Some removal on dialysis

FENTANYL § IV ok for acute management; short-acting § Transdermal: Never in an opioid-naïve patient § Possible accumulation of metabolites § Use low starting dose (12. 5 -25 mcg) and slow titration with patch (no more than one increase weekly) § Absorption affected in patients with low muscle mass, cachexia

OTHER CONSIDERATIONS WITH OPIOIDS § Bowel regimen § Cognitive impairment § Drowsiness § Pruritis § Dose requirements: offer Narcan (naloxone)

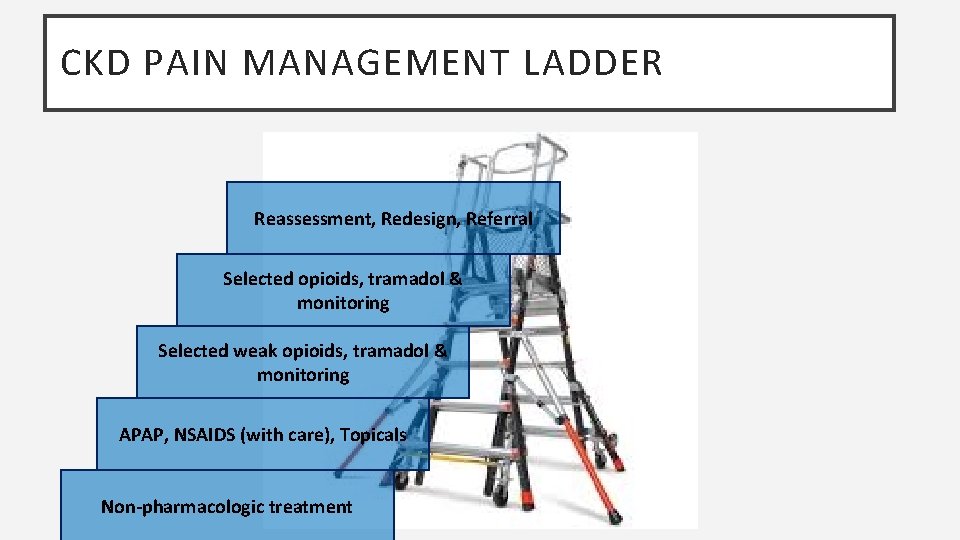
CKD PAIN MANAGEMENT LADDER Reassessment, Redesign, Referral Selected opioids, tramadol & monitoring Selected weak opioids, tramadol & monitoring APAP, NSAIDS (with care), Topicals Non-pharmacologic treatment

CONCLUSION § Pain is a common comorbidity in CKD patients affecting quality of life § Unique characteristics of CKD patients make effective and safe management of pain challenging § Thoughtful use of non-pharmacologic, non-opioid and opioid agents, including ongoing reassessment of care plan is essential to optimize pain management in this complex patient population

NON-OPIOID/OPIOID MEDICATION REFERENCE § Koncicki HM, et al. Pain Management in CKD: A Guide for Nephrology Providers. AJKD. 2017; 69(3): 451 -460. § Nagar VR, et al. Opioid Use in Chronic Pain Patients with CKD: A Systematic Review. Pain Medicine. 2017; 18 1416 -1449. § Pham PC, et al. 2017 Update on Pain Management in Patients with CKD. Clinical Kidney Journal. 2017; 10(5): 688 -697.

CMS ROADMAP TO ADDRESS THE OPIOID EPIDEMIC https: //www. cms. gov/About-CMS/Agency-Information/Emergency/Downloads/Opioid-epidemicroadmap. pdf