Tipologie di Audit e loro caratteristiche Riunione sottogruppo































- Slides: 32

Tipologie di Audit e loro caratteristiche Riunione sottogruppo GCP-GIQAR 21 Marzo 2006 Francesca Bucchi



PROCEDURES 1 - Preparing for the Audit 2 - Conducting the Audit 3 - Reporting the Audit 15 working days Draft Report Issued 15 working days Responses Provided Responses Reviewed/Accepted Final Report Issued



Responsibilities • Lead Auditor: Will facilitate organising, conducting and reporting of the audit. He/she will lead the audit activities and will prepare the audit report • PSA (Project Specific Auditor) In line with Line Management is responsible for: - Development of the audit programme - Being the focal point of contact with the clinical teams for audits - Ensuring that auditors conducting audits are aware of project related audit issues

Responsibilities (cont’d) • Process Owner: He/she is responsible for Harmonisation of all operational activities of the process, including its development and improvement

SYSTEMS AUDITS

What are Global Systems Audits? A systems audit is a review of the organisation, procedures and documentation related to a selected system, or an examination of a process or group of processes that result in an end product.

Why a “systems” approach ? • All work is process driven and the systems approach focuses on auditing the process • It can act as key fact finding tool for process improvement • It can provide an evaluation of interfaces within Sponsor, and between Sponsor and preferred providers • It can assess of cross-functional consistency in a global organisation

Some of the key areas for which a systems approach would be adopted include: • • Process Management and Training Trial Management Monitoring Data Management Safety Management Investigational Product (IP) Management Archiving of Essential Documents Computerised systems

How are they performed? Systems audits may consist of visit-based audits covering a number of locations or desk-based from the CQA offices. The audits may be conducted as a combination of documentation review, questionnaires and/or Interview

Audit Procedure Preparation • Assign Audit Team • Identify Audit Sponsor (global/local) • Define scope of the audit • Define audit references and standards • Develop audit plan & tools (e. g. audit method questionnaires, checklists, agenda) • Identify audit sample • Notification – early enough to ensure that personnel involved are available • Request for documentation

Audit Procedure (cont’d) Conducting the Audit • Audit may involve visits to one or more Sponsor sites or may be conducted via telephone discussions and review of documents only • Duration – between 2 and 4 days per site • Balance between discussions, review of documents and review of facilities • Discussions – encouraging staff to explain their daily work, understanding the issue & root cause • Focus is not on the individual • Review of documents – e. g. procedures, training records, CVs, job descriptions, documents related to the system audited • Review of facilities – e. g. archive

Audit Procedure (cont’d) Reporting and Follow-up • Initial feedback at the end of the audit • Draft audit report issued to Audit Sponsor for responding • Final report includes responses and actions • Audit Sponsor responsible for follow-up audit findings

Investigator Site Audits ABC HOSPITAL

Why Investigator Sites? • Obligation to ensure that studies are conducted to the relevant national and international laws • Obligation to ensure that studies are conducted to GCP • Assurance that the company and investigator sites would stand up to a regulatory inspection

Investigator Site Audits • Which studies? – regulatory submission status – priority of the project in R&D portfolio – pivotal status of study • Which centres? – 1 - 15%

Investigator Site Audits • Selection of investigator sites is based on: üRecruitment üSite workload üSite with new investigator, monitoring staff or using new systems üCoordinating investigator sites üRequests from study teams/compliance concerns (“For cause”)

Investigator Site Audits How are they performed? • Examination of 3 basic aspects – Nature of the investigator’s conduct of the study – The interaction between the monitor and the investigator – Patient source data that supports entries on the CRF • In 2 steps – At monitor’s office – At study site

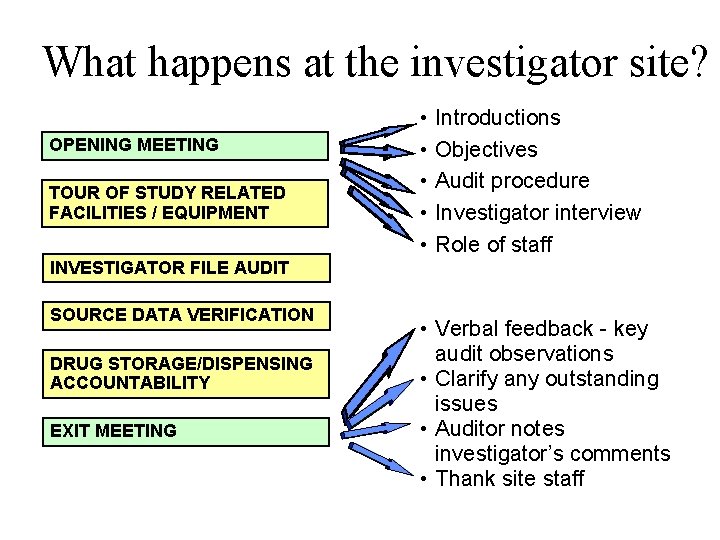
What happens at the investigator site? OPENING MEETING TOUR OF STUDY RELATED FACILITIES / EQUIPMENT • • • Introductions Objectives Audit procedure Investigator interview Role of staff INVESTIGATOR FILE AUDIT SOURCE DATA VERIFICATION DRUG STORAGE/DISPENSING ACCOUNTABILITY EXIT MEETING • Verbal feedback - key audit observations • Clarify any outstanding issues • Auditor notes investigator’s comments • Thank site staff

DOCUMENT AUDITS

Scope of Document Audits The document audits refer but are not limited to: - High Level Documents (HLDs) (integrated summary of data across studies for a particular section of a regulatory submission) - Investigator’s Brochures (IBs) - Clinical Study Protocols (CSPs) - Clinical Study Reports (CSRs) The quality of the document is assessed for internal consistency and against appropriate source documents, further to GCP and applicable regulatory requirements

How are they performed? • Review against relevant clinical SOPs, guidelines, templates, etc. • Check for completeness and logic • A review of the text for accuracy and consistency • A review against the source

DATABASE AUDITS

SCOPE • It is limited to the information in the clinical study database and the supporting documentation for the process from database set up to clean file • The audit of one database is divided into blocks and may be staged over time

PROCEDURE • Audit Structure It will be conducted in 3 blocks: 1) To verify presence and approval of a selection of DM documentation for the study 2) To verify data with clean data status from data entry site 3) To verify data that have completed central validation and to verify selected DM documentation

PROCEDURE • Electronic Data Capture For studies using Web Based Data Capture the procedure will be adjusted by omitting the verification of clean data from blocks 2 and 3

Audit of Clinical Research Organisations and Clinical Laboratories

This audit covers but is not limited to: • • • Clinical Research Organisations (CROs) Academic Research Organisations Site Management Organisations Collaborative Research Groups Clinical Laboratories (Labs) Other external provides of services such as IVRS, e-clinical technologies, etc. • Clinical Pharmacology Units (CPUs)

SCOPE OF THE AUDIT It may be one of 3 types: • Initial assessment audit of capabilities before work is transferred to the CRO/Lab • Follow-up of assessment of ongoing activities • For-cause audit where specific problems have been highlighted