Thymoquinone attenuates cisplatin induced toxicity and oxidative damage









- Slides: 19

Thymoquinone attenuates cisplatin induced toxicity and oxidative damage in rat kidney By: Zeba Farooqui Department of Biochemistry Aligarh Muslim University, India Supervisor Dr. Farah Khan

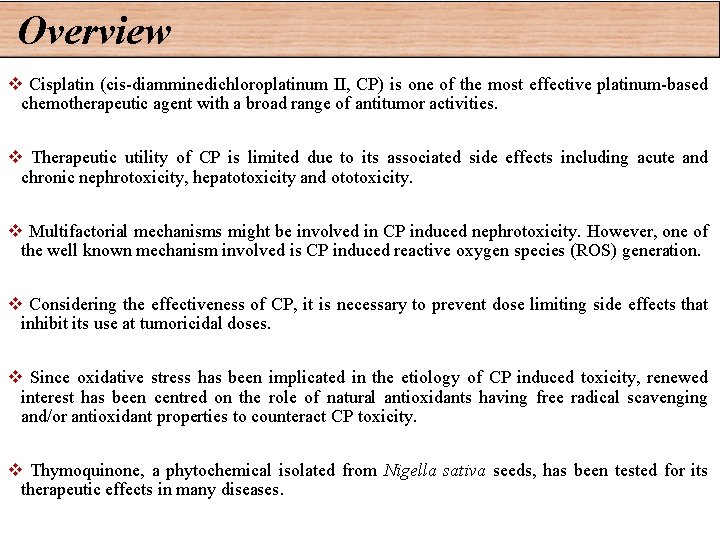
Overview v Cisplatin (cis-diamminedichloroplatinum II, CP) is one of the most effective platinum-based chemotherapeutic agent with a broad range of antitumor activities. v Therapeutic utility of CP is limited due to its associated side effects including acute and chronic nephrotoxicity, hepatotoxicity and ototoxicity. v Multifactorial mechanisms might be involved in CP induced nephrotoxicity. However, one of the well known mechanism involved is CP induced reactive oxygen species (ROS) generation. v Considering the effectiveness of CP, it is necessary to prevent dose limiting side effects that inhibit its use at tumoricidal doses. v Since oxidative stress has been implicated in the etiology of CP induced toxicity, renewed interest has been centred on the role of natural antioxidants having free radical scavenging and/or antioxidant properties to counteract CP toxicity. v Thymoquinone, a phytochemical isolated from Nigella sativa seeds, has been tested for its therapeutic effects in many diseases.

INTRODUCTION

Cisplatin v Inorganic complex of platinum, synthesized by Michael Peyrone in 1845. v Effectiveness as anticancer drug was discovered by Rosenberg and co workers in 1969. v Approved by Food and Drug Administration (FDA) in 1978. v Used for the treatment of various human solid tumors including those of head, neck, ovary, testes and breast. v Health risks: nephropathy, liver damage, hearing loss, myelosuppression, nausea and vomiting.

Pathophysiology of cisplatin in kidney v Adverse morphological changes in the S 3 subsegment of renal proximal tubule v Loss of brush border membrane v Alters membrane permeability v Depletes intracellular glutathione and interact with enzyme/protein sulphydryl groups v Perturbs antioxidant defense system v Tubular necrosis

Protection and/or prevention of cisplatin induced toxicity Strategies to ameliorate cisplatin induced toxicity Antioxidants: Flaxseed oil, Fish oil, Nigella sativa oil, melatonin, selenium, curcumin, silymarin, rutin

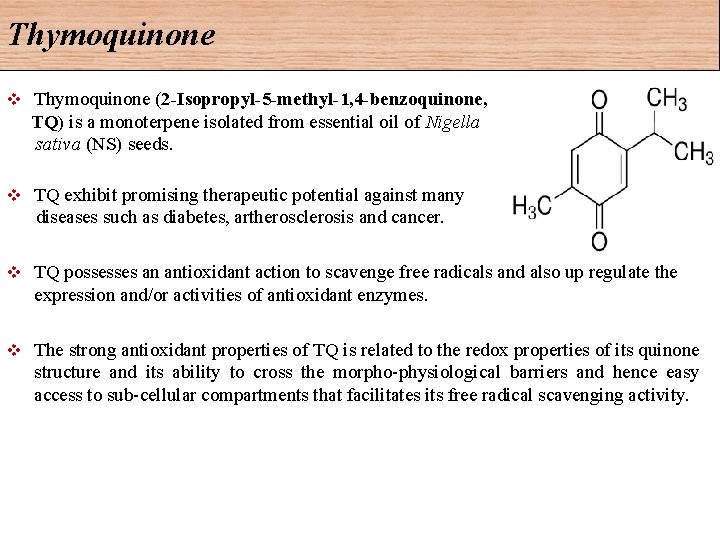
Thymoquinone (2 -Isopropyl-5 -methyl-1, 4 -benzoquinone, TQ) is a monoterpene isolated from essential oil of Nigella sativa (NS) seeds. v TQ exhibit promising therapeutic potential against many diseases such as diabetes, artherosclerosis and cancer. v v TQ possesses an antioxidant action to scavenge free radicals and also up regulate the expression and/or activities of antioxidant enzymes. v The strong antioxidant properties of TQ is related to the redox properties of its quinone structure and its ability to cross the morpho-physiological barriers and hence easy access to sub-cellular compartments that facilitates its free radical scavenging activity.

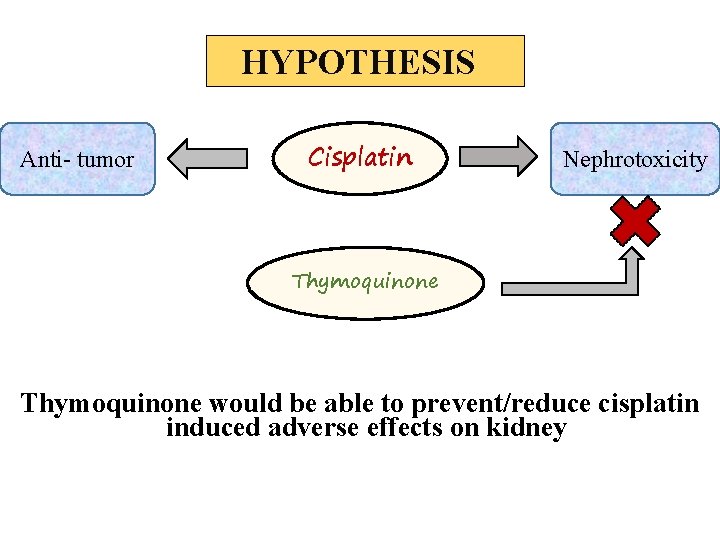
HYPOTHESIS Anti- tumor Cisplatin Nephrotoxicity Thymoquinone would be able to prevent/reduce cisplatin induced adverse effects on kidney

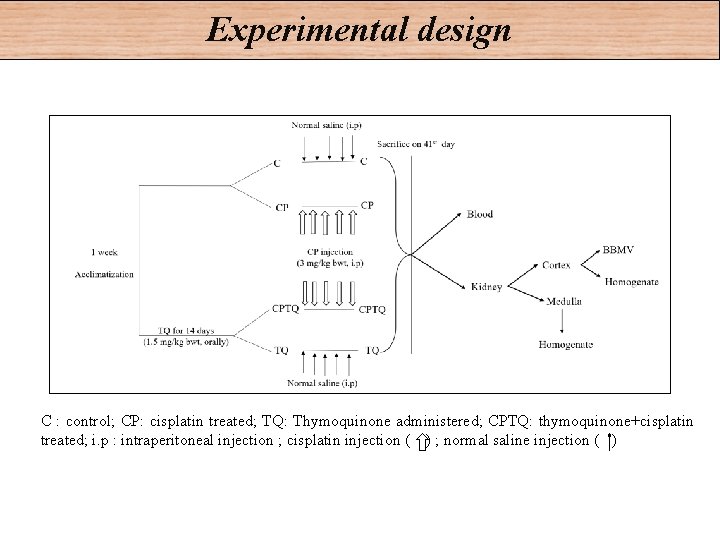
Experimental design C : control; CP: cisplatin treated; TQ: Thymoquinone administered; CPTQ: thymoquinone+cisplatin treated; i. p : intraperitoneal injection ; cisplatin injection ( ) ; normal saline injection ( )

Experiments were conducted to study the effect of CP alone and in combination with TQ on o various biochemical parameters in serum o enzymes of brush border membrane (BBM) in cortical and medullary homogenates and in cortical BBM vesicles (BBMV) ovarious oxidative stress parameters in cortical and medullary homogenates o histopathological examination of rat kidney

RESULTS

Table 1. Effect of TQ administration with and without CP treatment on serum parameters Groups Creatinine (mg/dl) 0. 93 ± 0. 03 1. 85 ± 0. 29* (+98. 92%) BUN (mg/dl) 12. 83 ± 1. 265 24. 21 ± 1. 773* (+88. 69%) Cholesterol (mg/dl) 54. 39 ± 2. 64 70. 06 ± 3. 61* (+28. 81%) Phospholipid (mg/dl) 116. 20 ± 5. 72 211. 98 ± 6. 28* (+82. 42%) Phosphate (μmols/ml) 2. 50 ± 0. 10 1. 31 ± 0. 18* (-47. 6%) Glucose (mg/dl) 100. 42 ± 0. 45 74. 29 ± 1. 10* (-26. 02%) TQ 0. 99 ± 0. 064 (+6. 45%) 13. 11 ± 1. 65 (+2. 18%) 49. 92 ± 3. 15 (-2. 74%) 125. 9 ± 6. 71 (+8. 34%) 2. 22 ± 0. 21 (-11. 2%) 117. 26 ± 6. 69 (+16. 76%) CPTQ 1. 13 ± 0. 05† (+21. 50%) 15. 24 ± 1. 20† (+18. 78%) 62. 16 ± 2. 99 (+14. 28%) 173. 65 ± 7. 73*† (+49. 44%) 1. 91 ± 0. 38 (-23. 6%) 92. 48 ± 7. 43† (-7. 91%) Control CP CP: cisplatin treated; TQ: Thymoquinone administered; CPTQ: Thymoquinone administered + cisplatin treated; BUN: blood urea nitrogen. Results are mean ± SEM for three different preparations. *Significantly different from control. † Significantly different from CP at p<0. 05 by one way ANOVA. Values in parenthesis represent percent change from control

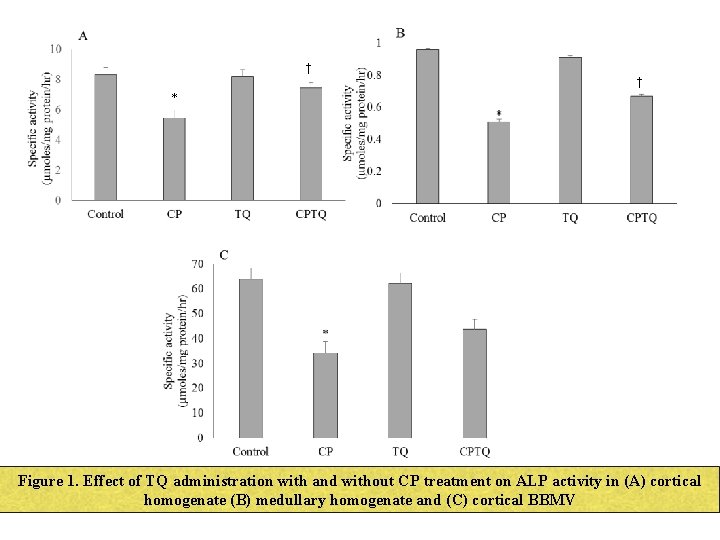
† † * Figure 1. Effect of TQ administration with and without CP treatment on ALP activity in (A) cortical homogenate (B) medullary homogenate and (C) cortical BBMV

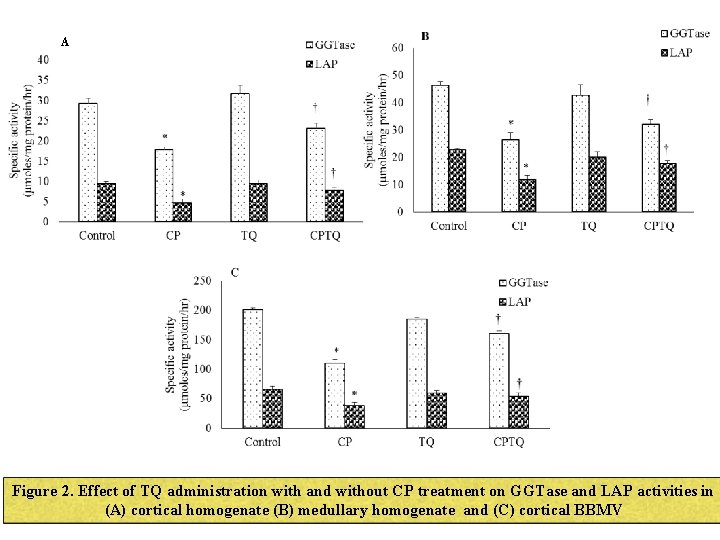
A Figure 2. Effect of TQ administration with and without CP treatment on GGTase and LAP activities in (A) cortical homogenate (B) medullary homogenate and (C) cortical BBMV

A B Figure 3. Effect of TQ administration with and without CP treatment on non enzymatic antioxidant parameters in the homogenates of (A) cortex and (B) medulla

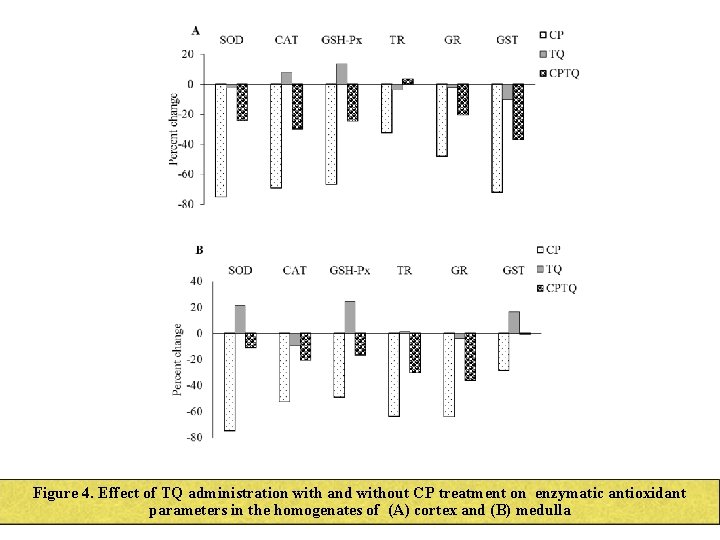
Figure 4. Effect of TQ administration with and without CP treatment on enzymatic antioxidant parameters in the homogenates of (A) cortex and (B) medulla

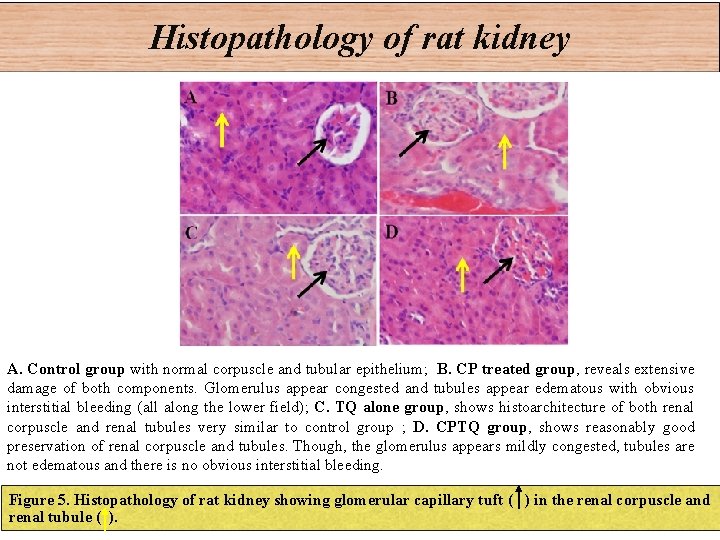
Histopathology of rat kidney A. Control group with normal corpuscle and tubular epithelium; B. CP treated group, reveals extensive damage of both components. Glomerulus appear congested and tubules appear edematous with obvious interstitial bleeding (all along the lower field); C. TQ alone group, shows histoarchitecture of both renal corpuscle and renal tubules very similar to control group ; D. CPTQ group, shows reasonably good preservation of renal corpuscle and tubules. Though, the glomerulus appears mildly congested, tubules are not edematous and there is no obvious interstitial bleeding. Figure 5. Histopathology of rat kidney showing glomerular capillary tuft ( ) in the renal corpuscle and renal tubule ( ).

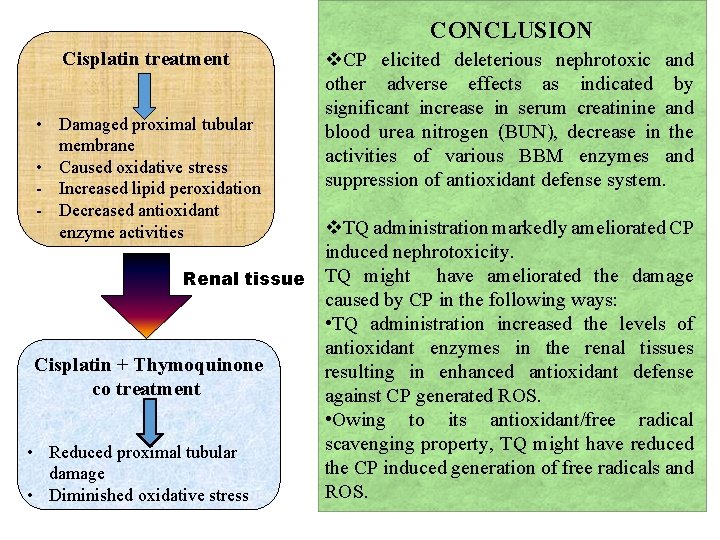
CONCLUSION Cisplatin treatment v. CP elicited deleterious nephrotoxic and other adverse effects as indicated by significant increase in serum creatinine and • Damaged proximal tubular blood urea nitrogen (BUN), decrease in the membrane activities of various BBM enzymes and • Caused oxidative stress suppression of antioxidant defense system. - Increased lipid peroxidation - Decreased antioxidant enzyme activities v. TQ administration markedly ameliorated CP induced nephrotoxicity. Renal tissue TQ might have ameliorated the damage caused by CP in the following ways: • TQ administration increased the levels of antioxidant enzymes in the renal tissues Cisplatin + Thymoquinone resulting in enhanced antioxidant defense co treatment against CP generated ROS. • Owing to its antioxidant/free radical scavenging property, TQ might have reduced • Reduced proximal tubular the CP induced generation of free radicals and damage ROS. • Diminished oxidative stress

THANK YOU
 London dispersion
London dispersion Oxygen toxicity
Oxygen toxicity Urea cycle definition
Urea cycle definition Transamination and oxidative deamination
Transamination and oxidative deamination Transamination and oxidative deamination
Transamination and oxidative deamination Induced fit model vs lock and key
Induced fit model vs lock and key B6 toxicity symptoms
B6 toxicity symptoms Mcgreafor
Mcgreafor Definition of chronic toxicity
Definition of chronic toxicity Vitamin d toxicity
Vitamin d toxicity Severe preeclampsia criteria
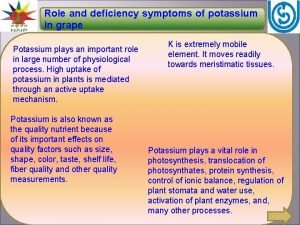
Severe preeclampsia criteria Symptoms of potassium
Symptoms of potassium What is oxygen toxicity
What is oxygen toxicity Organophosphate toxicity
Organophosphate toxicity Hepatic toxicity
Hepatic toxicity Lithium toxicity
Lithium toxicity Amiodarone thyroid toxicity
Amiodarone thyroid toxicity Health hazards pictogram
Health hazards pictogram Definition of chronic toxicity
Definition of chronic toxicity Digoxin mechanism
Digoxin mechanism