Thrombus management in the catheterization lab pharmacological options

Thrombus management in the catheterization lab: pharmacological options and outcomes George D. Dangas, MD, Ph. D Professor of Medicine (Cardiovascular Disease) The Icahn School of Medicine at Mount Sinai, New York, NY Cardiovascular Research Technologies - CRT 2015

Mount Sinai Department of Medicine Disclosure I receive financial support from the following company or companies related to the products listed below. These relationships may lead to bias in my presentation. Entity Type(s) of relationship(s) Abbott Vascular Advisory Board Boston Scientific Advisory Board Astra Zeneca Consultant/Scientific Advisory Board The Medicines Co. Speaker honoraria Research Grant Support (Institutional)

Thrombus in STEMI
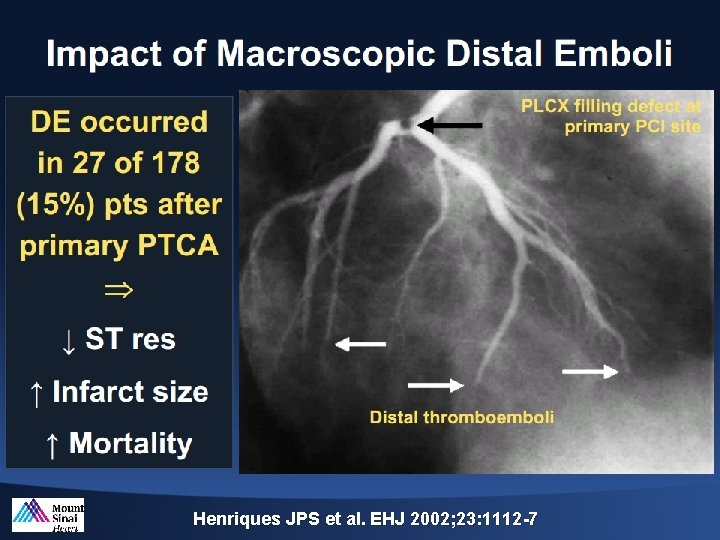
Henriques JPS et al. EHJ 2002; 23: 1112 -7

Impact of Thrombus Burden on Myocardial Damage Am J Cardiol 2014; 113: 1449 e 1456
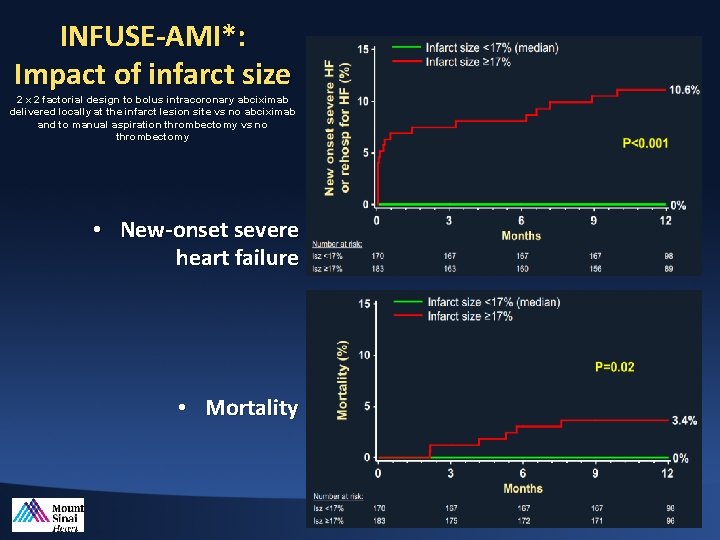
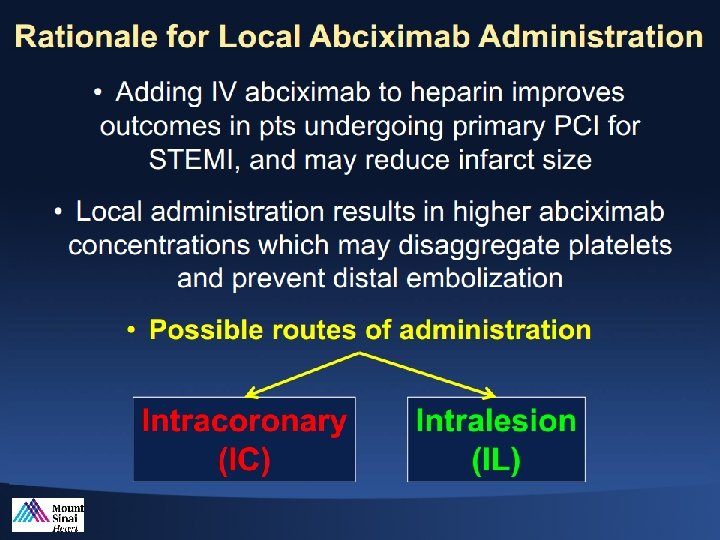
INFUSE-AMI*: Impact of infarct size 2 x 2 factorial design to bolus intracoronary abciximab delivered locally at the infarct lesion site vs no abciximab and to manual aspiration thrombectomy vs no thrombectomy • New-onset severe heart failure • Mortality

Thrombus Classification
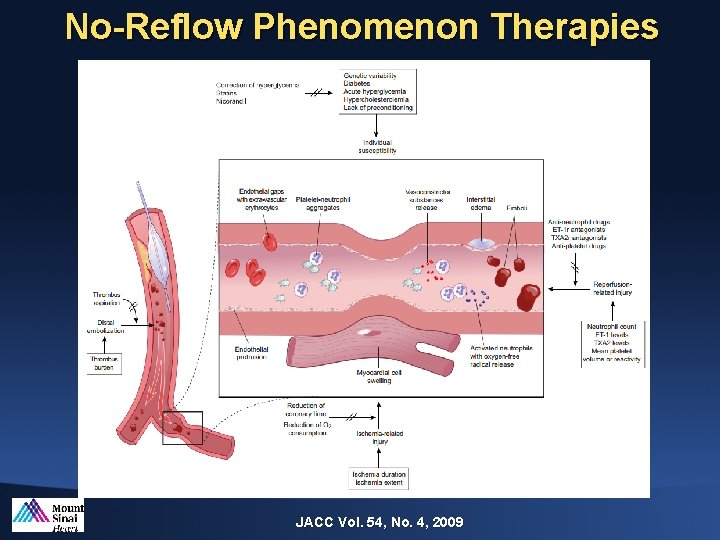
No-Reflow Phenomenon Therapies JACC Vol. 54, No. 4, 2009
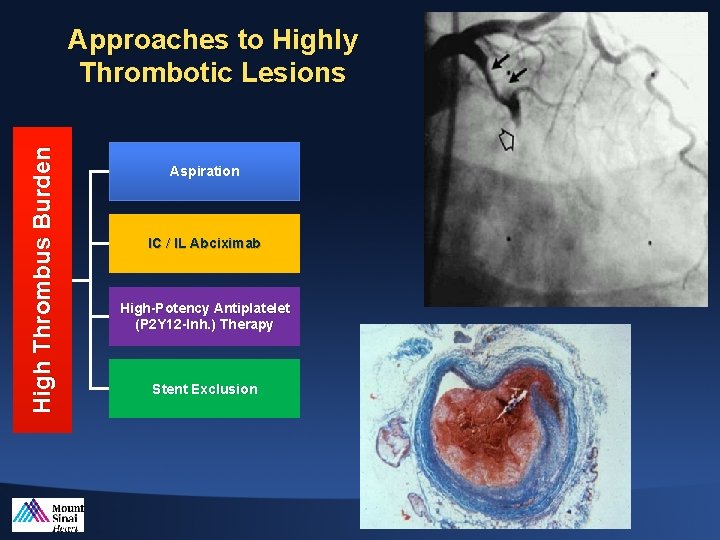
High Thrombus Burden Approaches to Highly Thrombotic Lesions Aspiration IC / IL Abciximab High-Potency Antiplatelet (P 2 Y 12 -Inh. ) Therapy Stent Exclusion
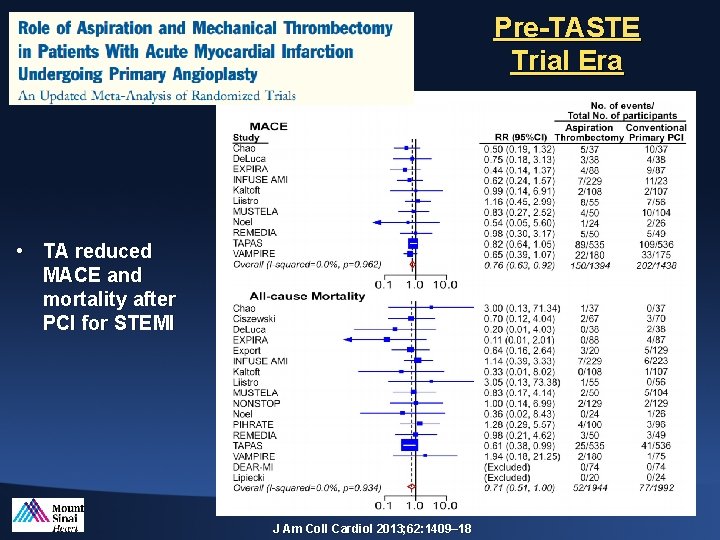
Pre-TASTE Trial Era • TA reduced MACE and mortality after PCI for STEMI J Am Coll Cardiol 2013; 62: 1409– 18
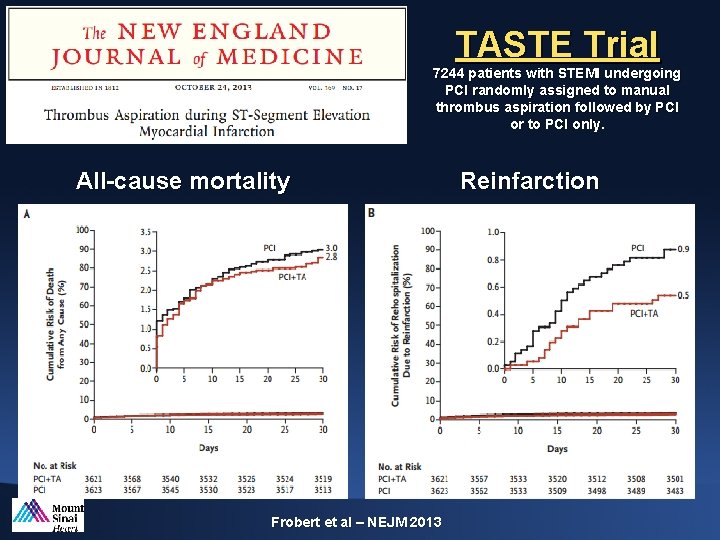
TASTE Trial 7244 patients with STEMI undergoing PCI randomly assigned to manual thrombus aspiration followed by PCI or to PCI only. All-cause mortality Frobert et al – NEJM 2013 Reinfarction
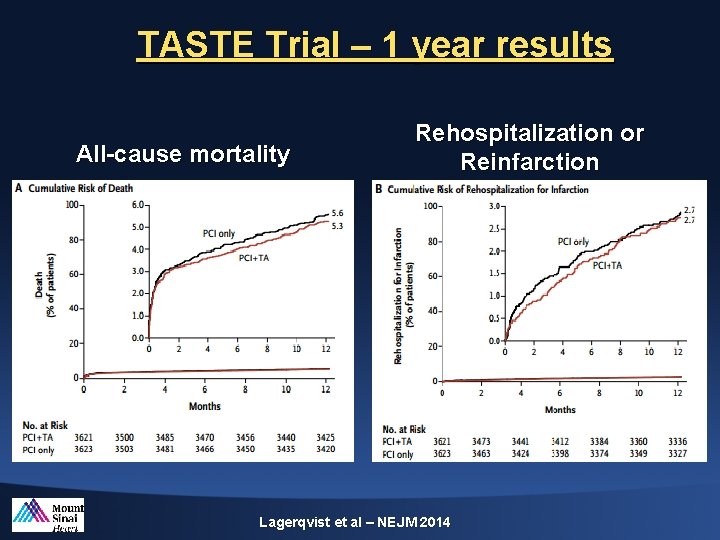
TASTE Trial – 1 year results All-cause mortality Rehospitalization or Reinfarction Lagerqvist et al – NEJM 2014
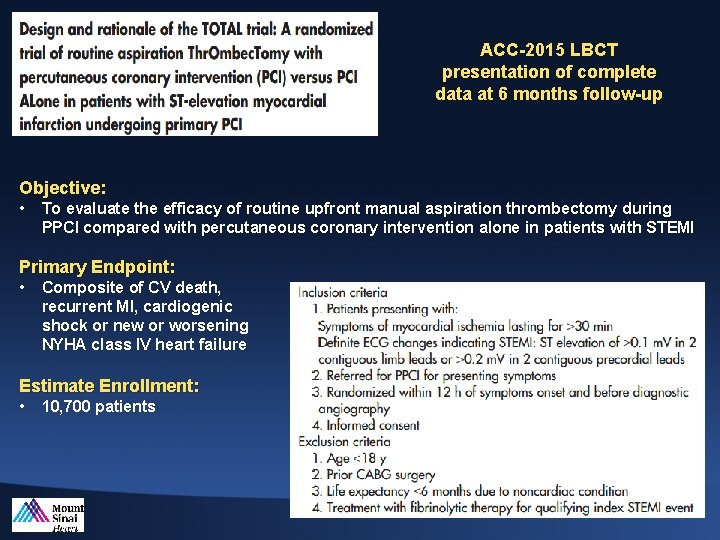
ACC-2015 LBCT presentation of complete data at 6 months follow-up Objective: • To evaluate the efficacy of routine upfront manual aspiration thrombectomy during PPCI compared with percutaneous coronary intervention alone in patients with STEMI Primary Endpoint: • Composite of CV death, recurrent MI, cardiogenic shock or new or worsening NYHA class IV heart failure Estimate Enrollment: • 10, 700 patients
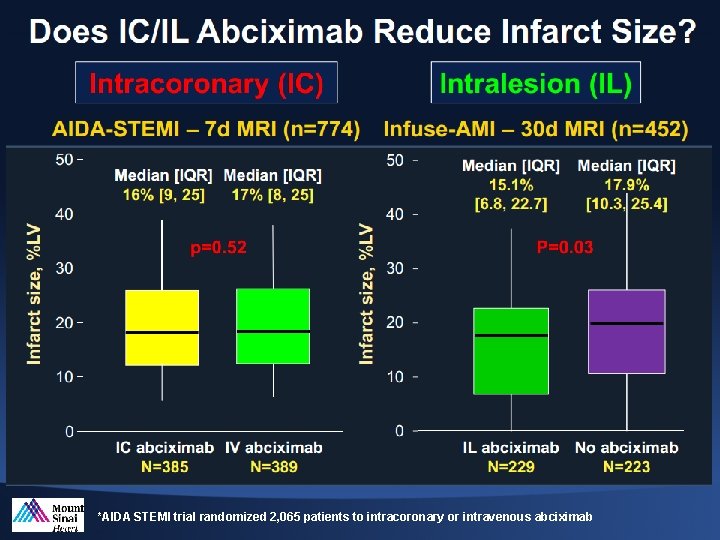
*AIDA STEMI trial randomized 2, 065 patients to intracoronary or intravenous abciximab

When should we use Abciximab? • When Aspirin and P 2 Y 12 -Inh. Fail or cannot be given before PCI for STEMI. • When emesis prevents the ingestion of oral APT agents or when cardiogenic shock, low output, or diabetic gastroparesis impairs gastrointestinal absorption, particularly in treatment-naïve patients. • In high-risk situations such as stent thrombosis or extensive intracoronary thrombus, IC abciximab may be preferable to IV abciximab. • Local delivery is preferable to guide-catheter infusion, which tends to blow drug back into the aorta or send it preferentially into the lower resistance circuit of nonculprit coronary arterial branches. • Local delivery (Stone – JAMA 2012) and guide-catheter infusion (Thiele – Lancet 2012) both constitute safe approaches for administering abciximab.
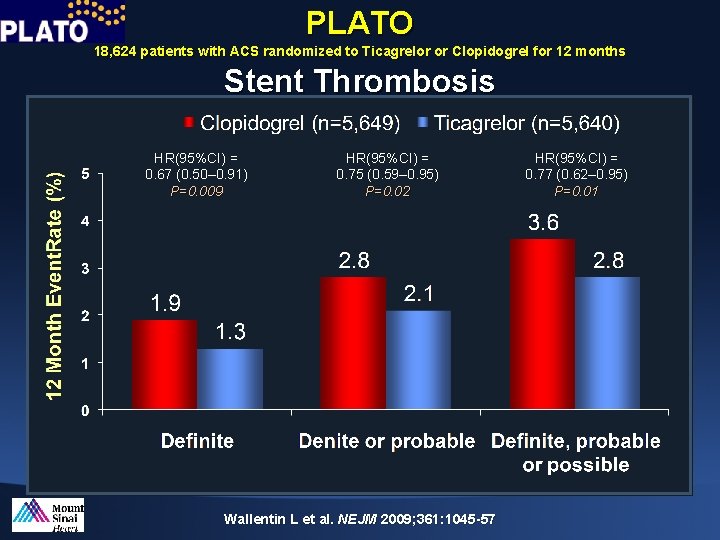
PLATO 18, 624 patients with ACS randomized to Ticagrelor or Clopidogrel for 12 months Stent Thrombosis HR(95%CI) = 0. 67 (0. 50– 0. 91) P=0. 009 HR(95%CI) = 0. 75 (0. 59– 0. 95) P=0. 02 Wallentin L et al. NEJM 2009; 361: 1045 -57 HR(95%CI) = 0. 77 (0. 62– 0. 95) P=0. 01
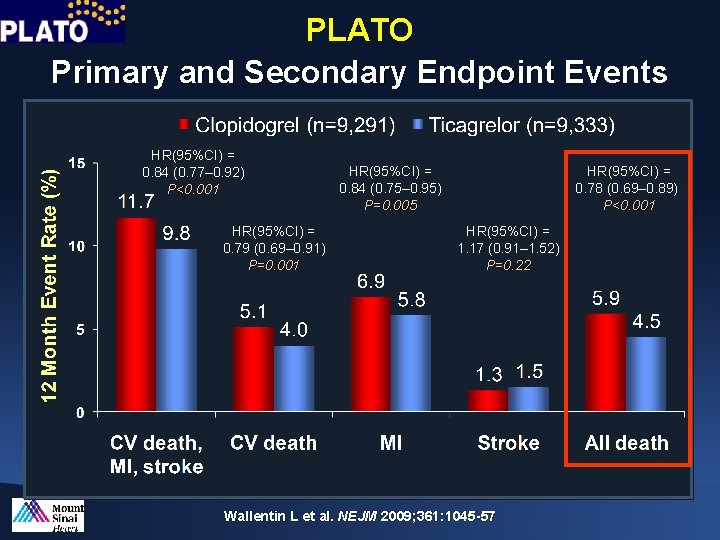
PLATO Primary and Secondary Endpoint Events HR(95%CI) = 0. 84 (0. 77– 0. 92) P<0. 001 HR(95%CI) = 0. 79 (0. 69– 0. 91) P=0. 001 HR(95%CI) = 0. 84 (0. 75– 0. 95) P=0. 005 HR(95%CI) = 0. 78 (0. 69– 0. 89)) P<0. 001 HR(95%CI) = 1. 17 (0. 91– 1. 52) P=0. 22 Wallentin L et al. NEJM 2009; 361: 1045 -57
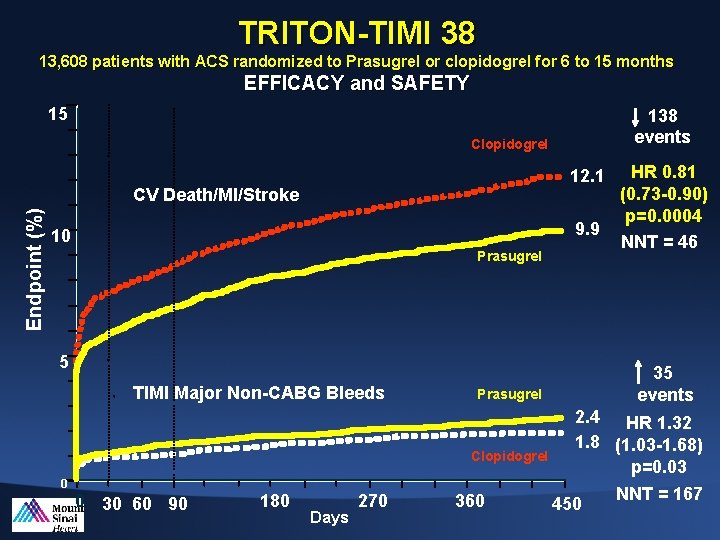
TRITON-TIMI 38 13, 608 patients with ACS randomized to Prasugrel or clopidogrel for 6 to 15 months EFFICACY and SAFETY 15 138 events Clopidogrel 12. 1 Endpoint (%) CV Death/MI/Stroke 9. 9 10 Prasugrel 5 TIMI Major Non-CABG Bleeds 35 events Prasugrel Clopidogrel 2. 4 HR 1. 32 1. 8 (1. 03 -1. 68) p=0. 03 0 0 30 60 90 180 Days 270 360 HR 0. 81 (0. 73 -0. 90) p=0. 0004 NNT = 46 450 NNT = 167
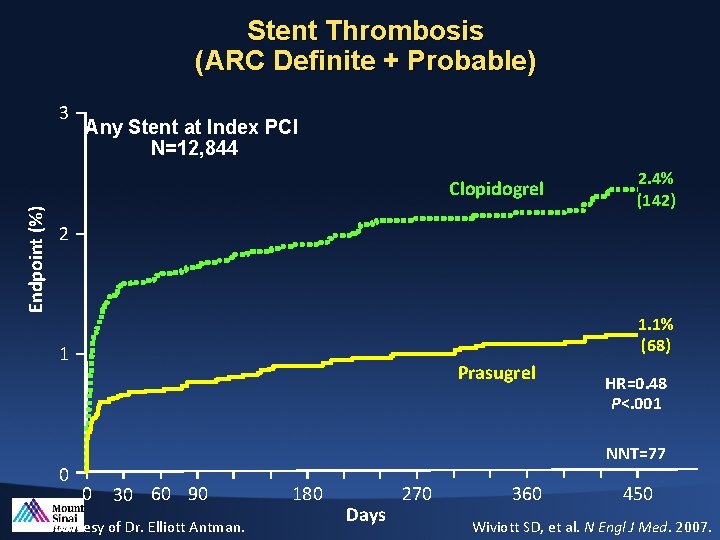
Stent Thrombosis (ARC Definite + Probable) 3 Any Stent at Index PCI N=12, 844 Endpoint (%) Clopidogrel 2. 4% (142) 2 1. 1% (68) 1 0 Prasugrel HR=0. 48 P<. 001 NNT=77 0 30 60 90 Slide courtesy of Dr. Elliott Antman. 180 Days 270 360 450 Wiviott SD, et al. N Engl J Med. 2007.
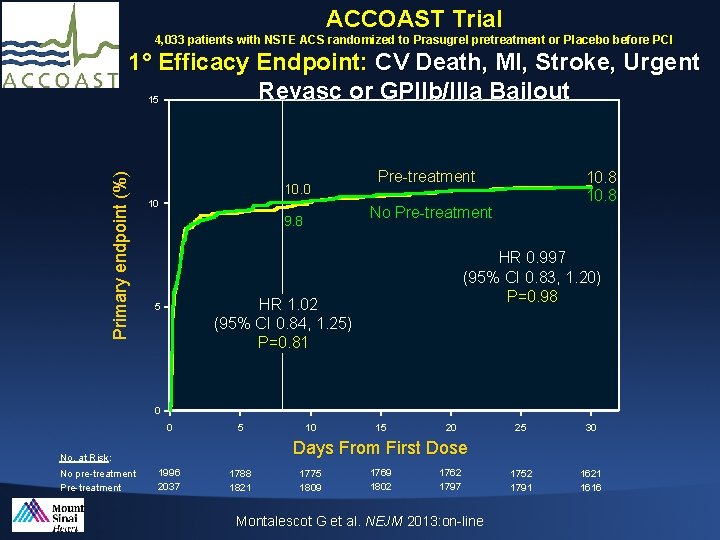
ACCOAST Trial 4, 033 patients with NSTE ACS randomized to Prasugrel pretreatment or Placebo before PCI Primary endpoint (%) 1° Efficacy Endpoint: CV Death, MI, Stroke, Urgent Revasc or GPIIb/IIIa Bailout 15 10. 0 10 Pre-treatment No Pre-treatment 9. 8 HR 0. 997 (95% CI 0. 83, 1. 20) P=0. 98 HR 1. 02 (95% CI 0. 84, 1. 25) P=0. 81 5 10. 8 0 0 5 15 20 25 30 1752 1791 1621 1616 Days From First Dose No. at Risk: No pre-treatment Pre-treatment 10 1996 2037 1788 1821 1775 1809 1769 1802 1762 1797 Montalescot G et al. NEJM 2013: on-line
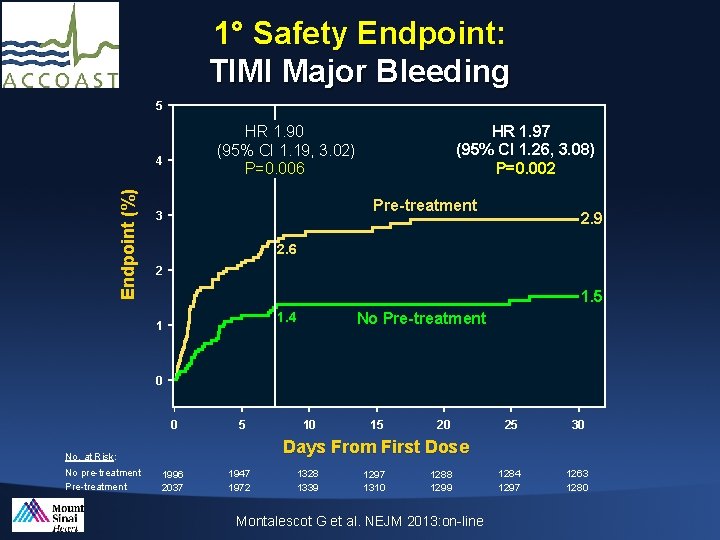
1° Safety Endpoint: TIMI Major Bleeding 5 4 Endpoint (%) HR 1. 97 (95% CI 1. 26, 3. 08) P=0. 002 HR 1. 90 (95% CI 1. 19, 3. 02) P=0. 006 Pre-treatment 3 2. 9 2. 6 2 1. 5 1. 4 1 No Pre-treatment 0 0 5 15 20 25 30 1284 1297 1263 1280 Days From First Dose : No. at Risk: No pre-treatment Pre-treatment 10 1996 2037 1947 1972 1328 1339 1297 1310 1288 1299 Montalescot G et al. NEJM 2013: on-line
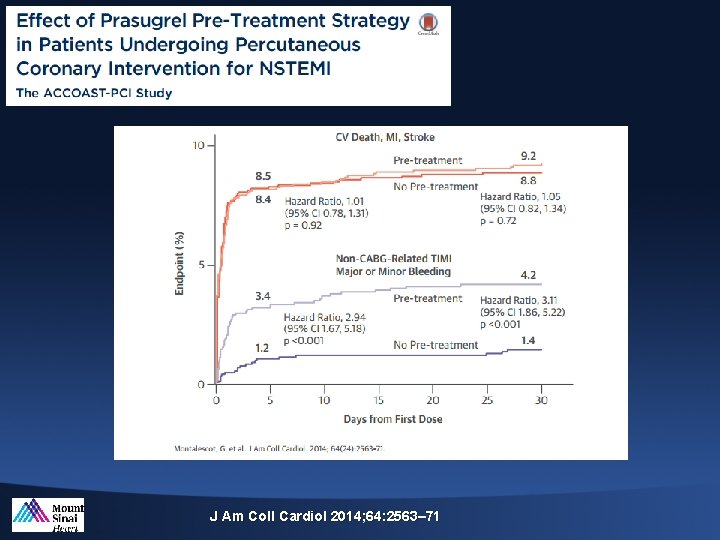
J Am Coll Cardiol 2014; 64: 2563– 71
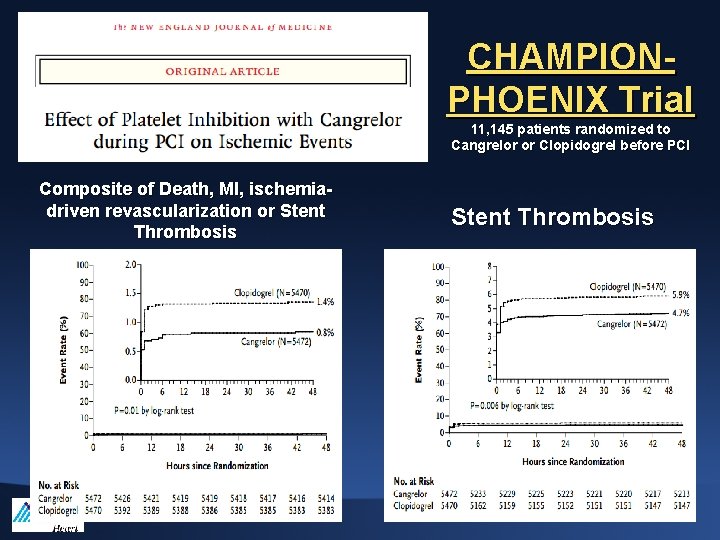
CHAMPIONPHOENIX Trial 11, 145 patients randomized to Cangrelor or Clopidogrel before PCI Composite of Death, MI, ischemiadriven revascularization or Stent Thrombosis
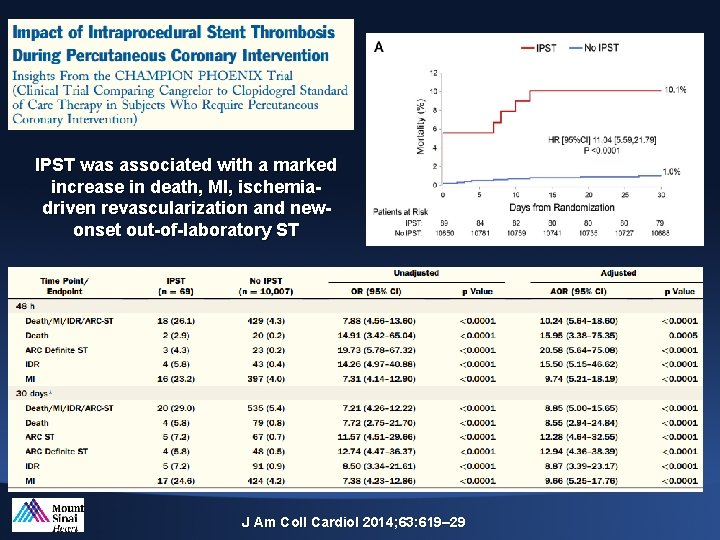
IPST was associated with a marked increase in death, MI, ischemiadriven revascularization and newonset out-of-laboratory ST J Am Coll Cardiol 2014; 63: 619– 29
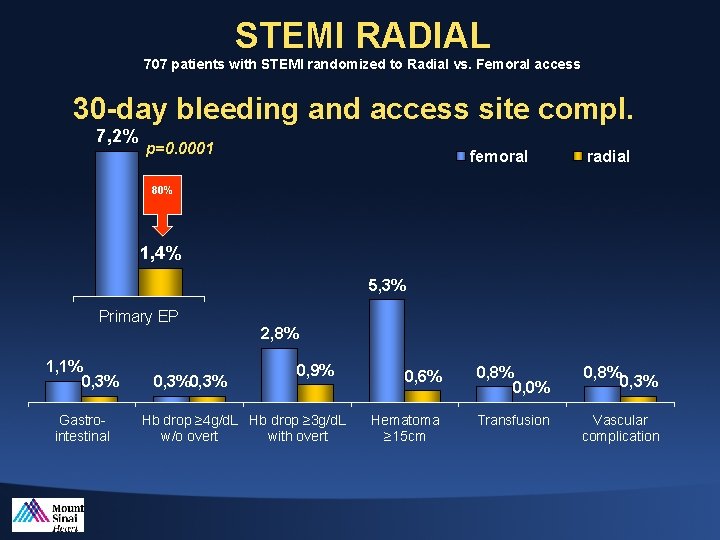
STEMI RADIAL 707 patients with STEMI randomized to Radial vs. Femoral access 30 -day bleeding and access site compl. 7, 2% p=0. 0001 femoral radial 80% 1, 4% 5, 3% Primary EP 1, 1% 0, 3% Gastrointestinal 0, 3% 2, 8% 0, 9% Hb drop ≥ 4 g/d. L Hb drop ≥ 3 g/d. L w/o overt with overt 0, 6% 0, 8% 0, 0% 0, 8% 0, 3% Hematoma ≥ 15 cm Transfusion Vascular complication
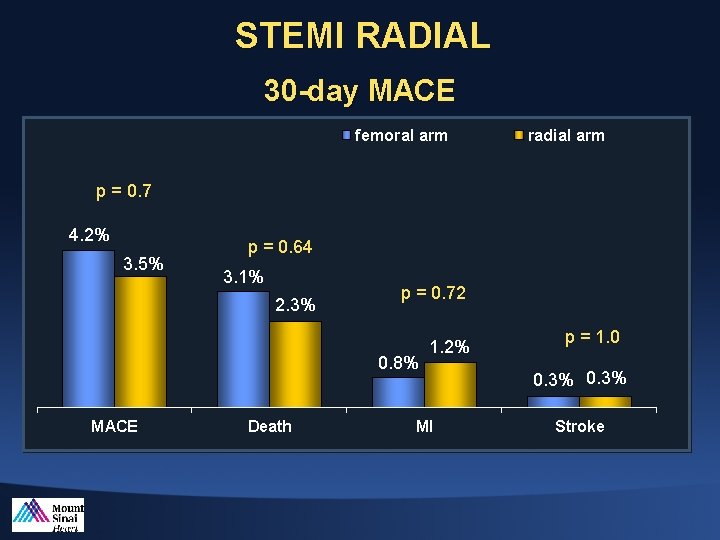
STEMI RADIAL 30 -day MACE femoral arm radial arm p = 0. 7 4. 2% 3. 5% p = 0. 64 3. 1% 2. 3% p = 0. 72 0. 8% MACE Death 1. 2% MI p = 1. 0 0. 3% Stroke
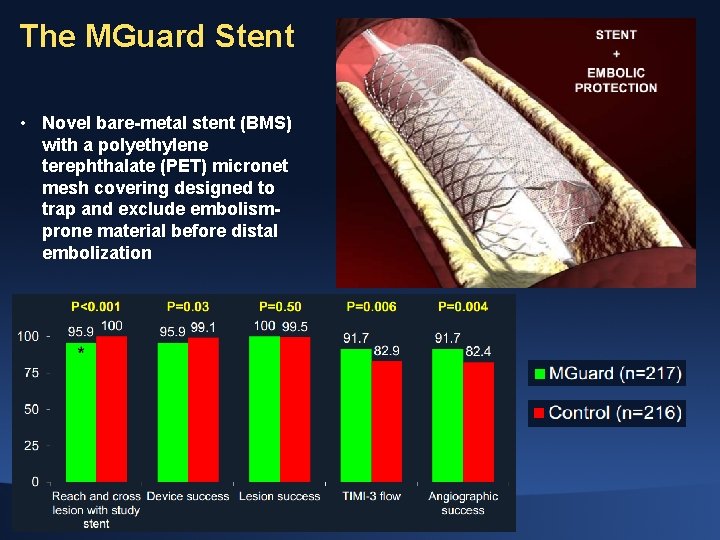
The MGuard Stent • Novel bare-metal stent (BMS) with a polyethylene terephthalate (PET) micronet mesh covering designed to trap and exclude embolismprone material before distal embolization

• Thank you for your attention
- Slides: 29