Three basic things we need to know about

• Three basic things we need to know about materials: (1) How do they behave in service? (2) Why do they behave in the way that they do? (3) Is there anything we can do to alter their behavior? • 1740 –Dalton et al formulated atomic theory • 1824 – Sadi Carnot proposed first scientific studies of materials change • The science of thermodynamics – the transfer of energy – energy of atoms, energy of the tides, energy of lifting rig etc • Engineering is all about compromise and trade-off. 1
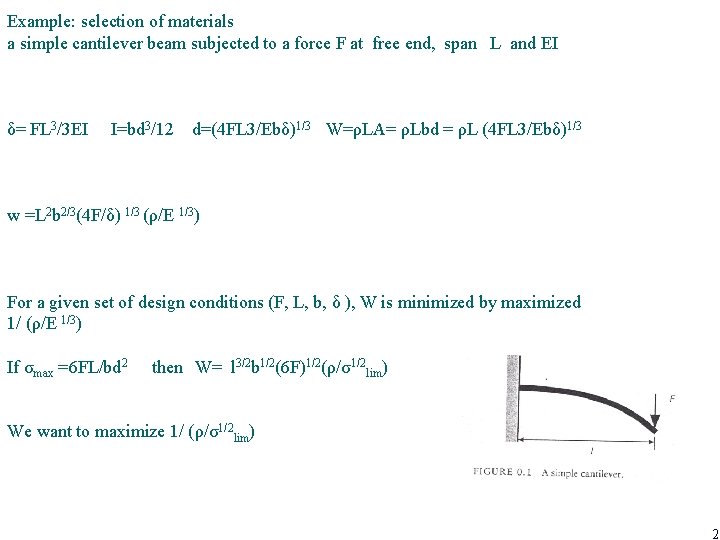
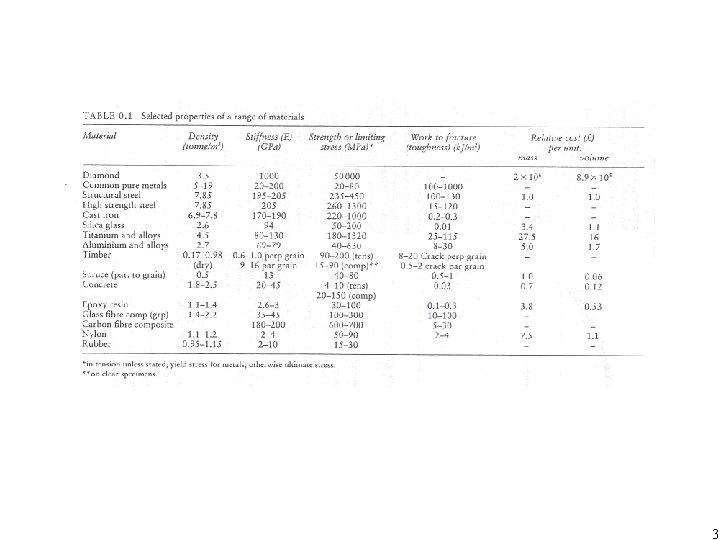
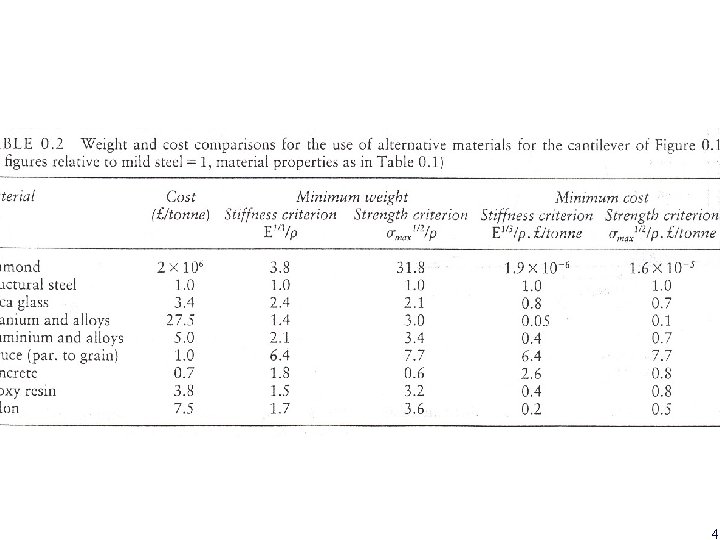
Example: selection of materials a simple cantilever beam subjected to a force F at free end, span L and EI δ= FL 3/3 EI I=bd 3/12 d=(4 FL 3/Ebδ)1/3 W=ρLA= ρLbd = ρL (4 FL 3/Ebδ)1/3 w =L 2 b 2/3(4 F/δ) 1/3 (ρ/E 1/3) For a given set of design conditions (F, L, b, δ ), W is minimized by maximized 1/ (ρ/E 1/3) If σmax =6 FL/bd 2 then W= l 3/2 b 1/2(6 F)1/2(ρ/σ1/2 lim) We want to maximize 1/ (ρ/σ1/2 lim) 2
3
4
5
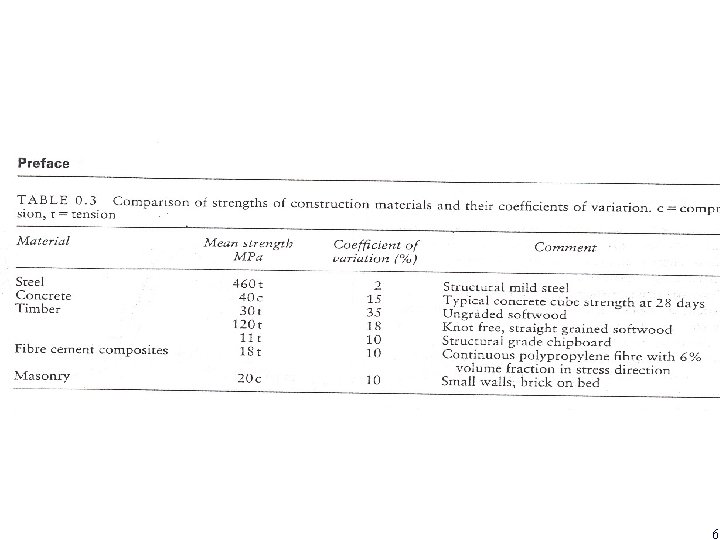
6
Introduction Handout #2 • Information from mechanical testing, experience in processing, handling and placing, and materials science, i. e. , empiricism, craft and science, be brought together to give the sound foundation for materials technology required by the practitioner. • Level of information: the molecular level – atomic model to form the physical structure, both regular or disordered structure. The size of the particles are in the range of 10 -7 to 10 -3 mm, crystal structure of metals, cellulose molecules in timber, calcium silicate hydrates in hardened cement paste and variety of polymers, such as PVC (polyvinyl chloride) , included in fiber composites Chemical composition – durability. Chemical and physical facrors also come together in determining whether or not the material is porous. 7

• Materials structural level 1. Geometry level – the shape, size and concentration of the particles and their distribution in the matrix or continuous phase. 2. State and properties – the chemical and physical properties of the individual phase influence the structure and behavior of the total material. 3. Interfacial effect – the interfaces between the phases may introduce additional modes of behavior that cannot be related to the individual properties of the phases. This is especially true of strength, the breakdown of the material often being controlled by the bond strength at an interface. 8

• The engineering level Cells for various materials: 10 -3 m for metals, 100 mm for concrete, and 1000 mm for masonry Selection of materials: Fitness-for-purpose Strength, deformation, and durability – the principal criteria must be satisfied. 9

The Science and Technology of Civil Engineering Materials VX 0255 Young‧‧Mindess‧‧Gray‧‧Bentur 10
All materials are made of atoms. Atoms are the smallest part of an element that still retain the properties of that element. These atoms are bonded together indifferent patterns using different methods to form different materials. • All Materials refer to four important categories: 1. chemical composition; 2 how the material occurs in nature; 3. the refining or manufacturing processes the material must undergo before it reaches its final or applicable state; 4. atomic structure. • Periodic table of elements: atom number, atomic mass, proton, electron, neutron, atom= nucleus + orbiting electrons • Wgt. of electron ≈1/2000 wgt. of proton • Isotopes: P: atomic number; N: neutron number; A( mass number) = P+N Elements have same P but Different A, which have same chemical properties; However some isotopes are unstable and radioactive. 11
Quantum mechanics • It is common to have more than one electron in an orbit, two electrons in the same orbit have the same energy content but they must spin in opposite direction, clockwise or counterclockwise direction. • Shell and subshell : 1 s, 2 s 2 p, 3 s 3 p 3 d, , (s: 2, p: 6, d: 10, f: 14) • The total energy of electrons is determined by the principal quantum number (n), second quantum number (angular momentum, l ), and the third quantum number (magnetic momentum, M). n=1, 2, 3, 4…… l=0 to n-1; the subshell is 2(2 l+1); M= -1 to +1; Ms: spin direction: ½ to - ½ No two electrons has the same four quantum numbers Starting with the fourth period, lower shell must be filled before the outer most energy shell is filled. 12
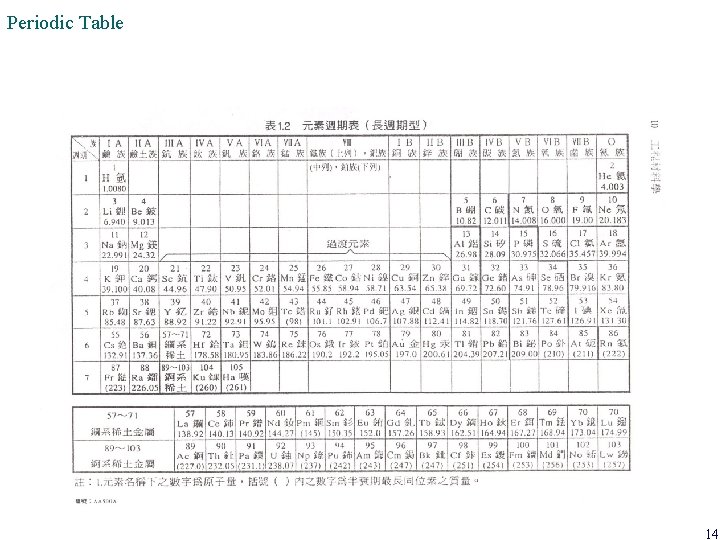
Periodic Table • The vertical groupings in the periodic table are based on similar electron configurations and similarities in both chemical and physical properties. • Group IA: alkali metal; Group IIA: alkaline-earth metal; Group IIIA thru VA, VIIA are mostly non -metals; the last group: inert gases • Elements can be divided into two groups: metal and non-metal • About 92 elements occur naturally in the earth; the total elements are about 120 nowaday 13
Periodic Table 14
• Compounds are combinations of two or more elements. Alloys, molecules • Mixtures are consisted of two or more pure substances that have been mechanically mixed l together. The pure substances can be elements or compounds. One example of a mixture is oil and water, which can be mixed into an emulsion and later separated. • Organic and inorganic material-carbon-based or noncarbon-based material 15
Introduction • The chemical bonds serve to ensure that atoms to achieve stable electron configurations by adding, removing, or sharing electrons. • The bonds can be categorized into two groups: strong (primary) bonds between atoms (ionic, covalent, and metallic) and weak (secondary) bond (van der Waals bond and hydrogen bond) between molecules. • The position of an element in the periodic table determines the type of chemical bond it can form l • Elements in Group I and Group II lose electrons to from cations (i. e. , they are strongly electropositive), while at the other end of the periodic table, elements in Group VI and Group VII readily gain electrons (they are strongly electronegative) • M+ X- M is a Group I element and X is a Group VII element • M+2 X-2 M is a Group II element and X is a Group VI element • 16

ionic bonds • The interaction energy between a pair of ions is proportional to (z+z-e 2)/r, where z is the ionic charge, e=4. 80 x 10 -10, and r is the distance between ions. • When materials gain or lose an electron, they become ions. Ions are versions of the original element that deviate from the normal neutral state-as for instance, when sodium and chlorine combine to form salt. The sodium atom relinquishes its valence electron and becomes a positive ion. Chlorine, with its seven outer electrons, absorbs this electron to become an ion. The sodium and chlorine share an electron. The attraction of the ions for the electron holds the two together. Neutral and stable bonding exists. 17


1. 2 IONIC BONDS 19
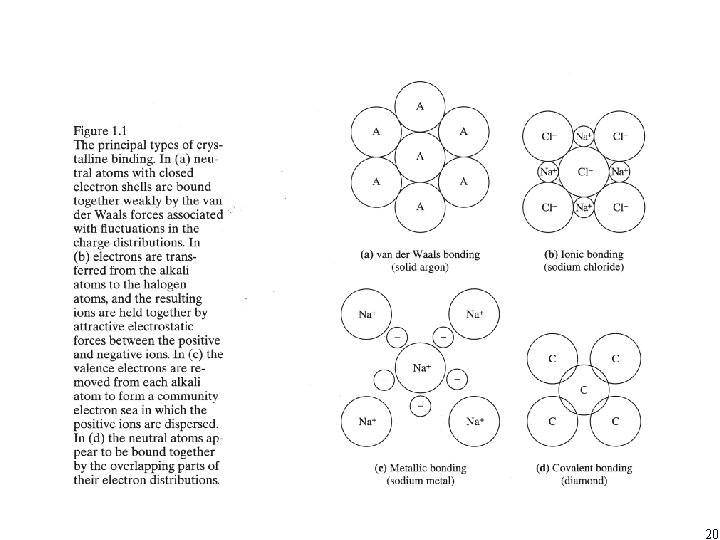
20
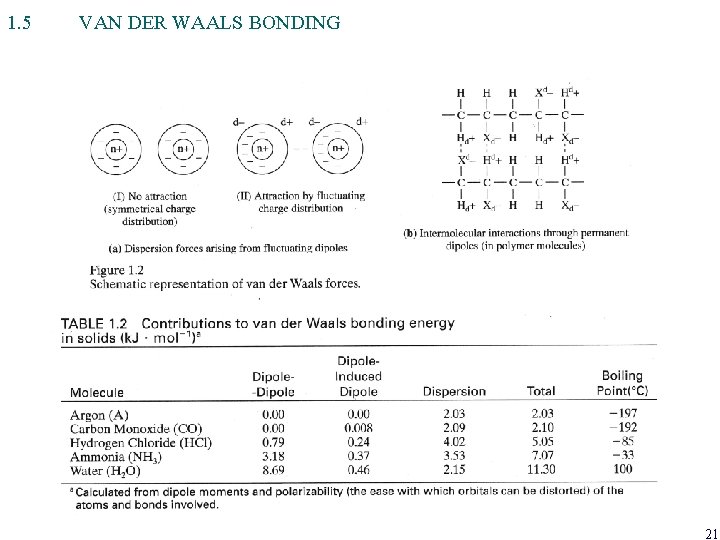
1. 5 VAN DER WAALS BONDING 21
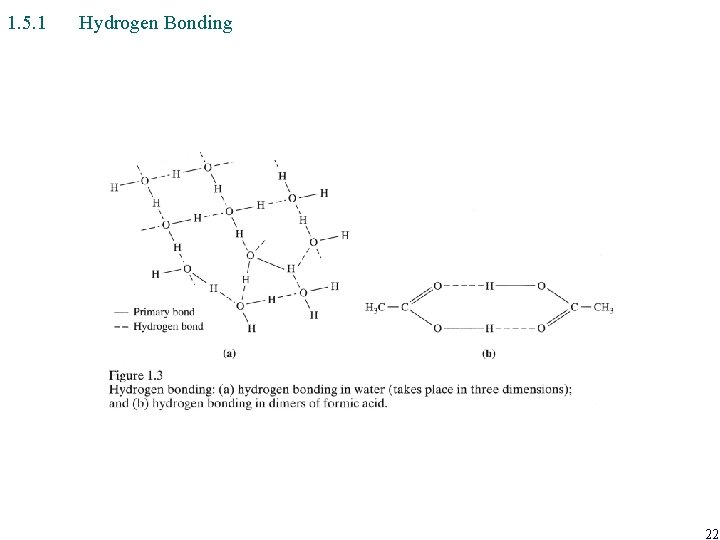
1. 5. 1 Hydrogen Bonding 22

1. 6 BONDING ENERGIES 23

24
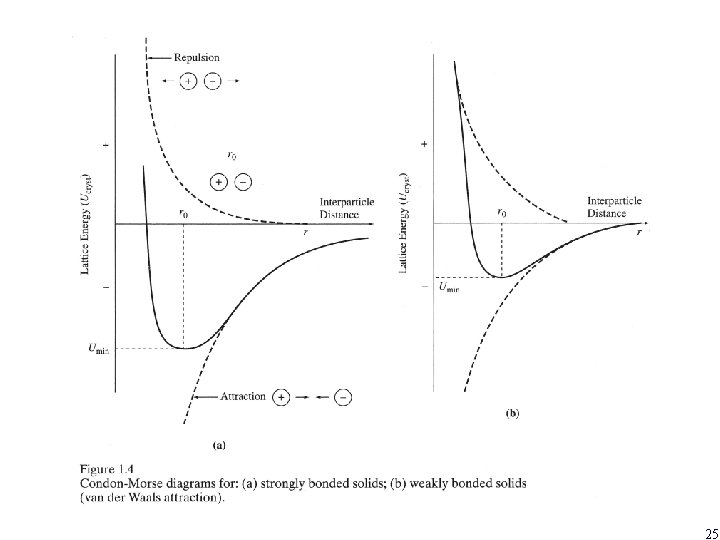
25
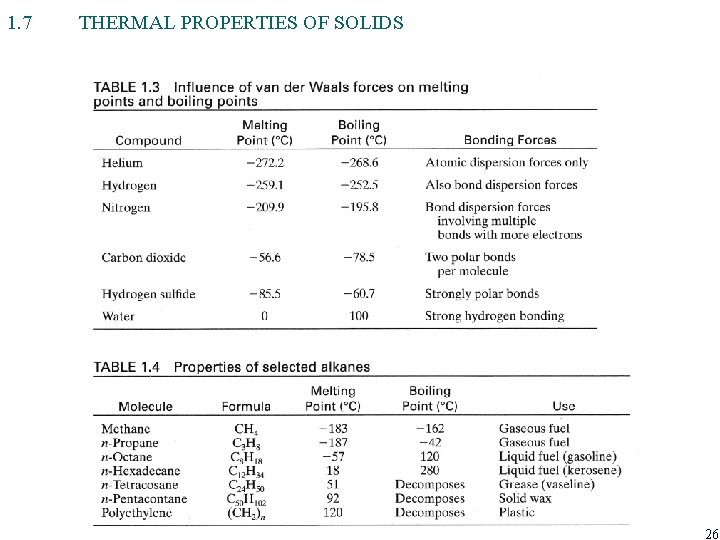
1. 7 THERMAL PROPERTIES OF SOLIDS 26

27
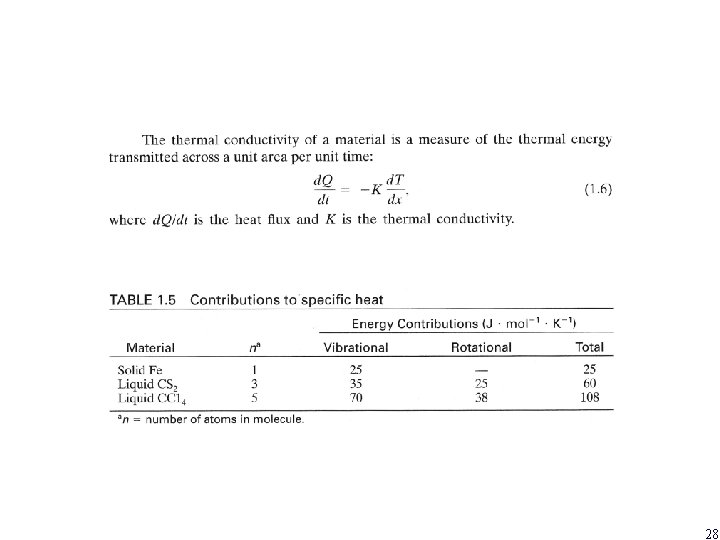
28
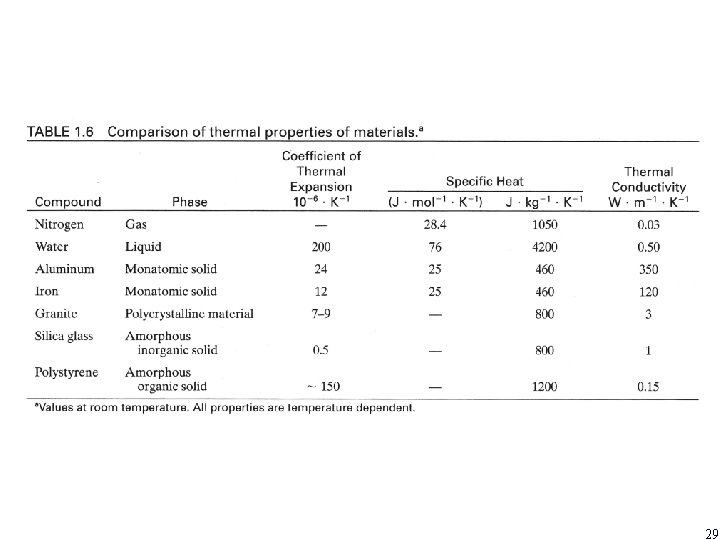
29

1. 8 BONDING FORCES 30
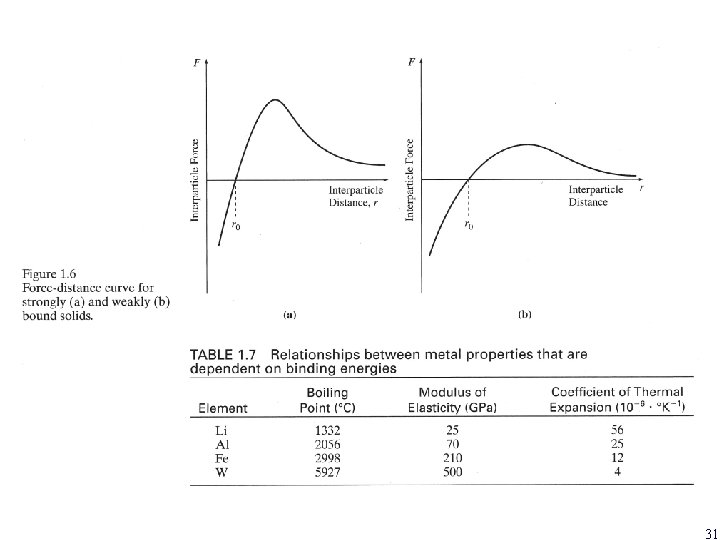
31

32
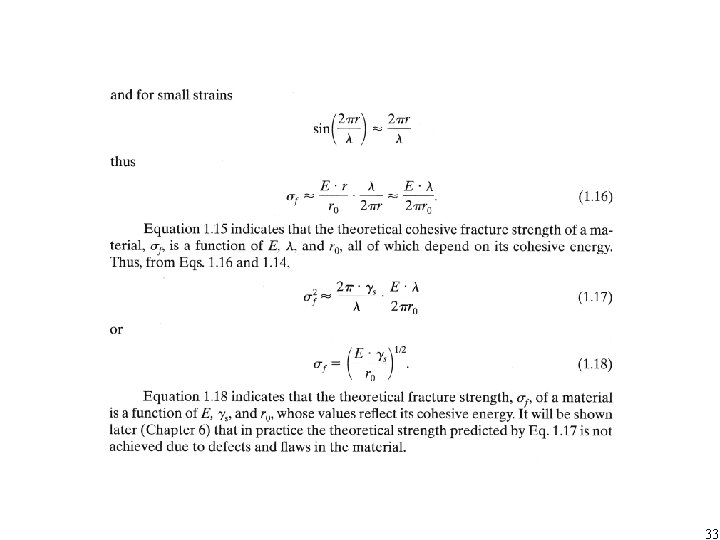
33
- Slides: 33