THINK What is matter Matter Think Pair Share

























- Slides: 74

THINK What is matter? Matter Think – Pair - Share SHARE What do you think? PAIR Individually, think of what you know about “matter”. This was part of your 6 th grade science class. Write down what comes to mind when you think of “matter”. 5 7 minutes Now pair up with a partner (or team) and share your thoughts and ideas. One person serve are the recorder and put these on the construction paper. 5 – 7 minutes Each team will then share their information with the class. Tape the construction paper up for other students to see.

Matter: Properties and Change http: //www. youtube. com/watch? v=FSy. Aeh. Mdpy. I “The Nucleus: Crash Course Introduction To Chemistry” #1” 10: 12 Matter, is a substance that has mass and also volume. The volume is determined by the space it occupies, while the mass is defined as a measure of how much matter is in an object. http: //micro. magnet. fsu. edu/primer/java/scienceopticsu/powersof 10/ size comparisons from milky way galaxy to quarks

What is matter? Explain your reasoning. http: //commons. wikimedia. org Sunlight Water Electricity Heat Smoke Earth’s Atmosphere

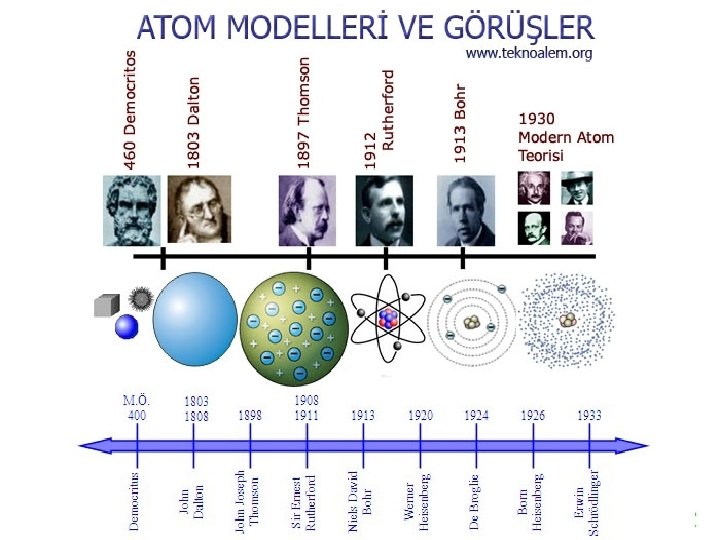
Early alchemists thought that everything was made of either earth, air, water or fire.


Why can you never trust an atom?

“The Elements” VHS

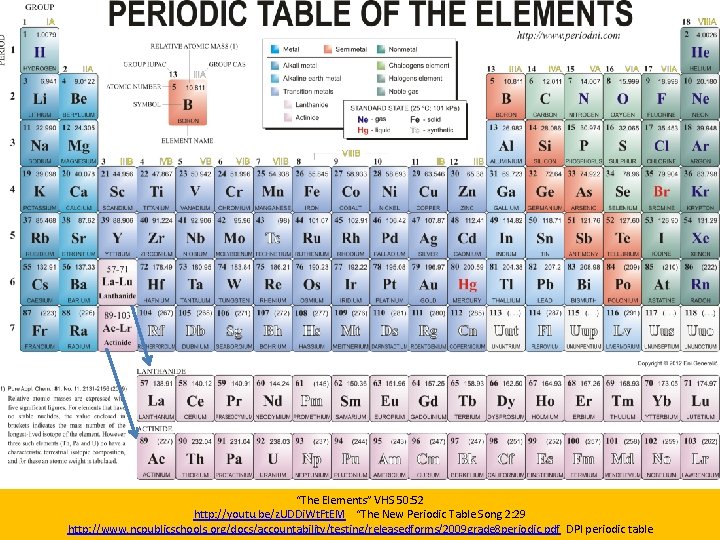
“The Elements” VHS 50: 52 http: //youtu. be/z. UDDi. Wt. Ft. EM “The New Periodic Table Song 2: 29 http: //www. ncpublicschools. org/docs/accountability/testing/releasedforms/2009 grade 8 periodic. pdf DPI periodic table

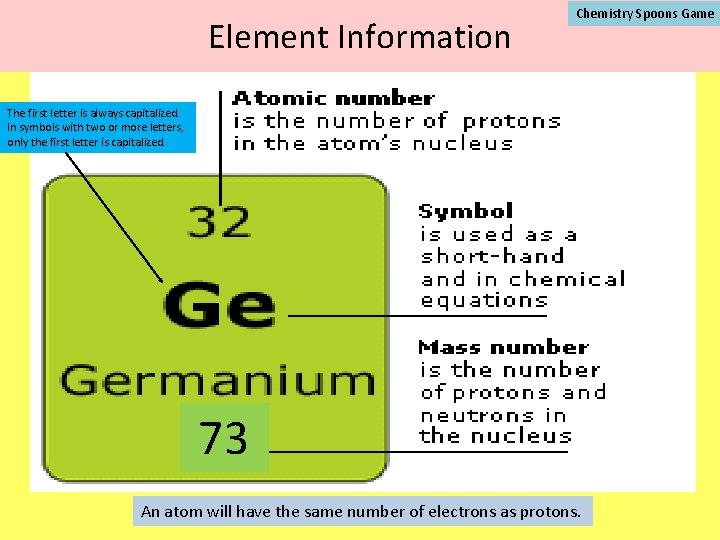
Element Information Chemistry Spoons Game The first letter is always capitalized. In symbols with two or more letters, only the first letter is capitalized. 73 An atom will have the same number of electrons as protons.

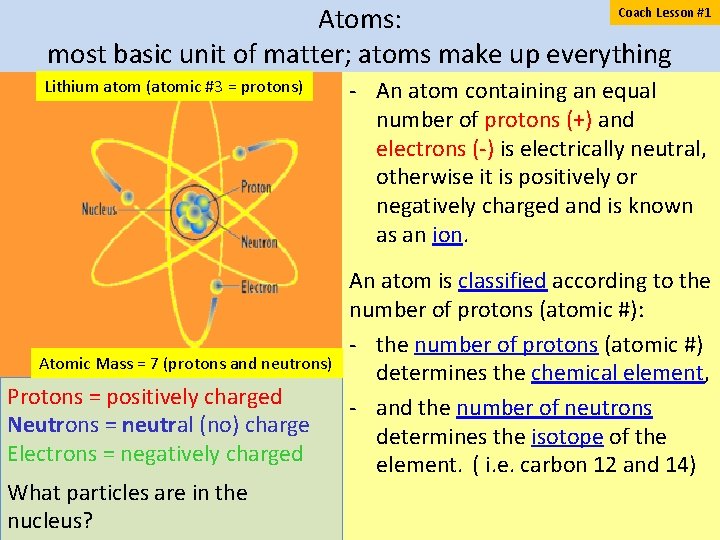
Coach Lesson #1 Atoms: most basic unit of matter; atoms make up everything Lithium atom (atomic #3 = protons) Atomic Mass = 7 (protons and neutrons) Protons = positively charged Neutrons = neutral (no) charge Electrons = negatively charged What particles are in the nucleus? An atom containing an equal number of protons (+) and electrons ( ) is electrically neutral, otherwise it is positively or negatively charged and is known as an ion. An atom is classified according to the number of protons (atomic #): the number of protons (atomic #) determines the chemical element, and the number of neutrons determines the isotope of the element. ( i. e. carbon 12 and 14)

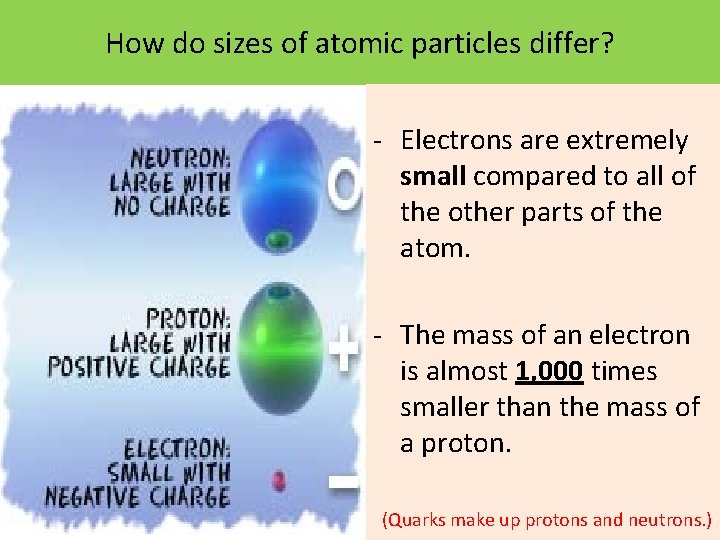
How do sizes of atomic particles differ? Electrons are extremely small compared to all of the other parts of the atom. The mass of an electron is almost 1, 000 times smaller than the mass of a proton. (Quarks make up protons and neutrons. )

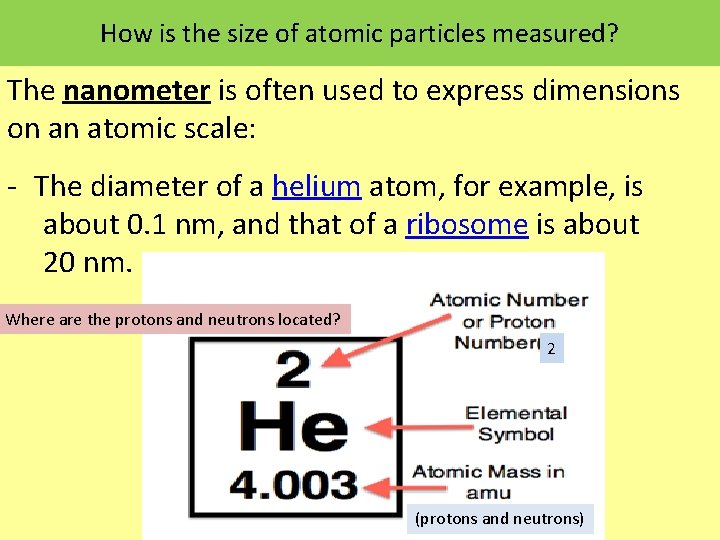
How is the size of atomic particles measured? The nanometer is often used to express dimensions on an atomic scale: The diameter of a helium atom, for example, is about 0. 1 nm, and that of a ribosome is about 20 nm. Where are the protons and neutrons located? 2 (protons and neutrons)

Electron Orbitals/ Cloud/Energy Level/Shell Configurations How many valence electrons are shown in this atom? Valence electrons are those located in the outer most shell/level.

Atomic Structure of Helium 1. 2. 3. 4. How many protons(+) are in helium? How many neutrons (neutral) are in helium? How many electrons (-) are in helium? How many valence (outer level) electrons are shown?

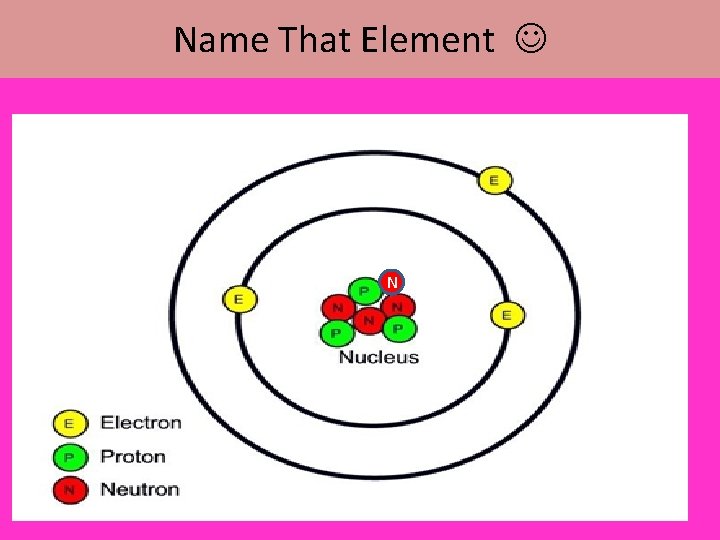
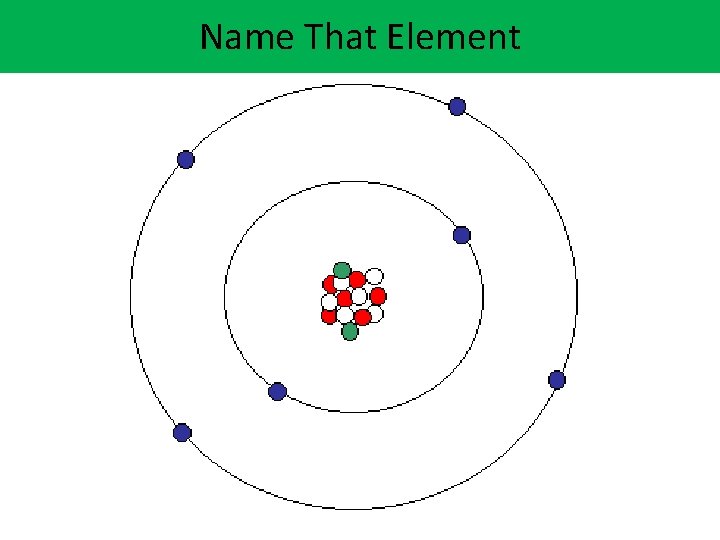
Name That Element N

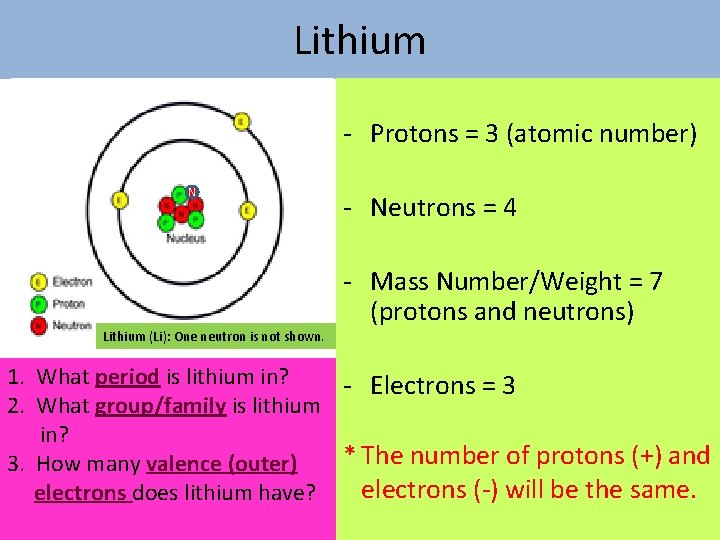
Lithium Protons = 3 (atomic number) N Lithium (Li): One neutron is not shown. Neutrons = 4 Mass Number/Weight = 7 (protons and neutrons) 1. What period is lithium in? Electrons = 3 2. What group/family is lithium in? * The number of protons (+) and 3. How many valence (outer) electrons ( ) will be the same. electrons does lithium have?

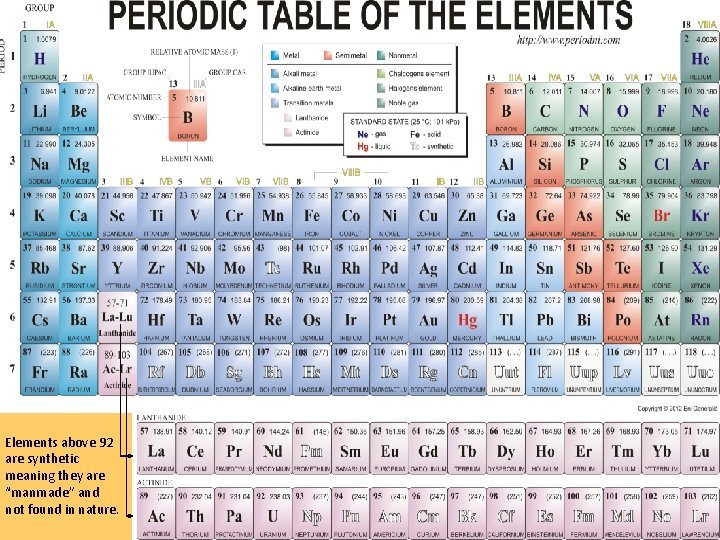
Elements above 92 are synthetic meaning they are “manmade” and not found in nature.

Name That Element

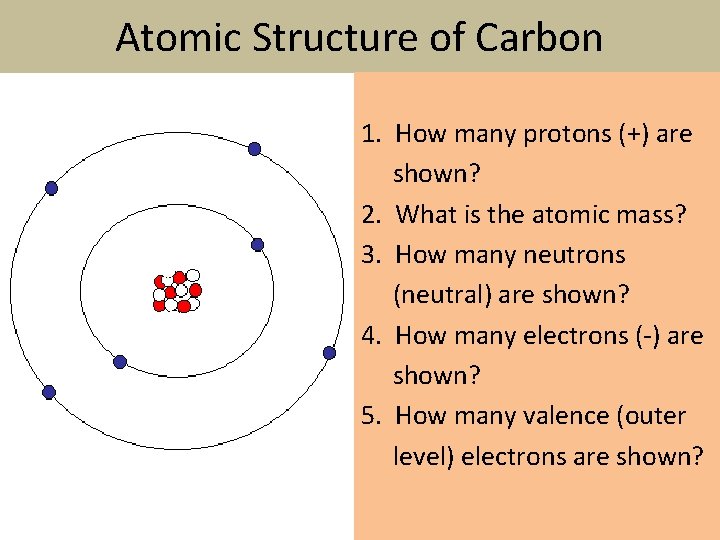
Atomic Structure of Carbon 1. How many protons (+) are shown? 2. What is the atomic mass? 3. How many neutrons (neutral) are shown? 4. How many electrons ( ) are shown? 5. How many valence (outer level) electrons are shown?

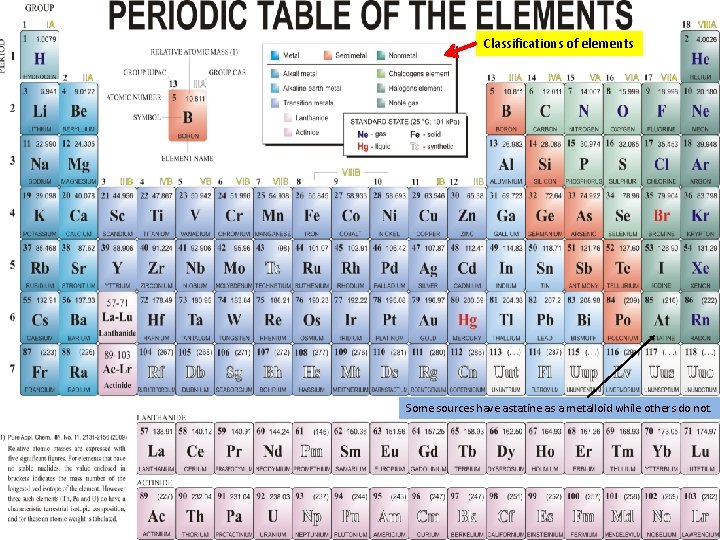
Classifications of elements Some sources have astatine as a metalloid while others do not.

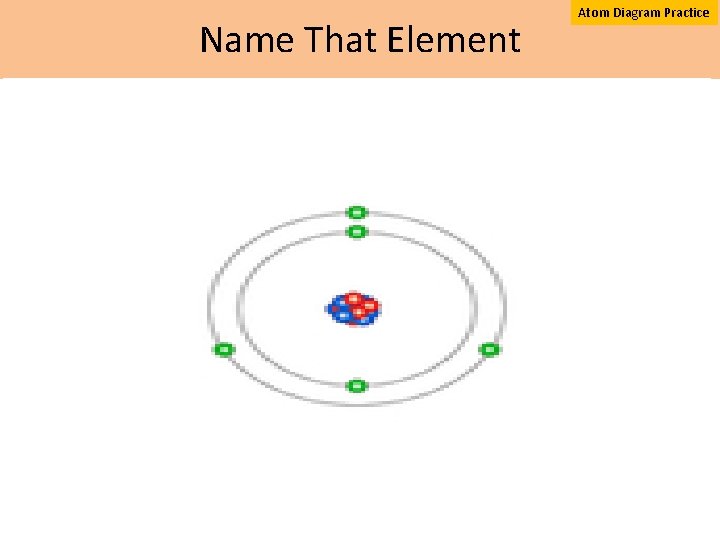
Name That Element Atom Diagram Practice


Atomic Structure of Boron “Build That Atom” Activity http: //phet. colorado. edu/en/simulation/build an atom activity A. What is the atomic number of boron? B. What is the mass number of boron? C. What family is boron in? D. What group is boron in? 1. How many protons (+) are shown? 2. How many neutrons (neutral) are shown? 3. How many electrons ( ) are shown? 4. How many valence (outer level) electrons are shown?

“The Elements” VHS 50: 52

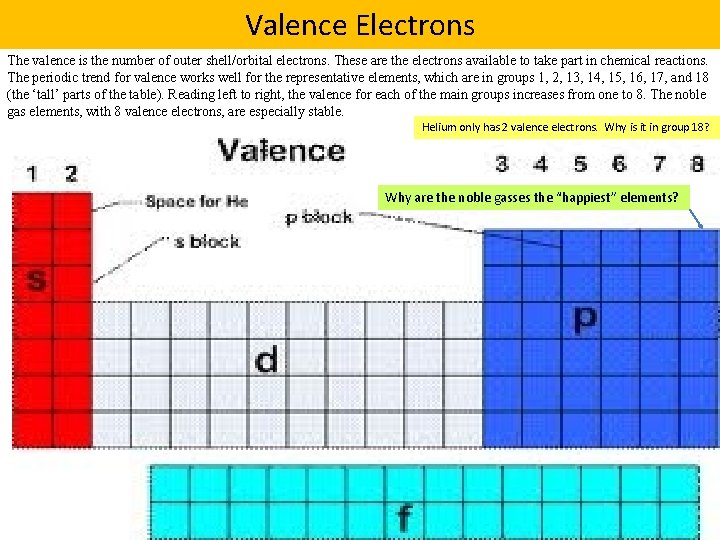
Valence Electrons The valence is the number of outer shell/orbital electrons. These are the electrons available to take part in chemical reactions. The periodic trend for valence works well for the representative elements, which are in groups 1, 2, 13, 14, 15, 16, 17, and 18 (the ‘tall’ parts of the table). Reading left to right, the valence for each of the main groups increases from one to 8. The noble gas elements, with 8 valence electrons, are especially stable. Helium only has 2 valence electrons. Why is it in group 18? Why are the noble gasses the “happiest” elements?

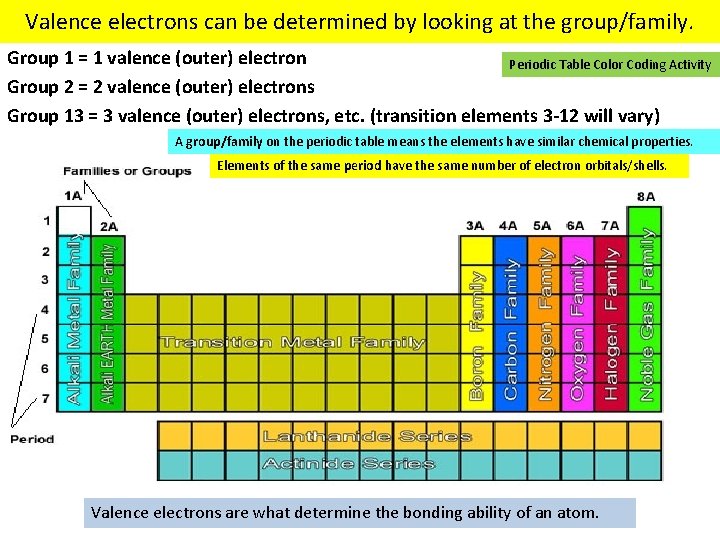
Valence electrons can be determined by looking at the group/family. Group 1 = 1 valence (outer) electron Periodic Table Color Coding Activity Group 2 = 2 valence (outer) electrons Group 13 = 3 valence (outer) electrons, etc. (transition elements 3 -12 will vary) A group/family on the periodic table means the elements have similar chemical properties. Elements of the same period have the same number of electron orbitals/shells. Valence electrons are what determine the bonding ability of an atom.

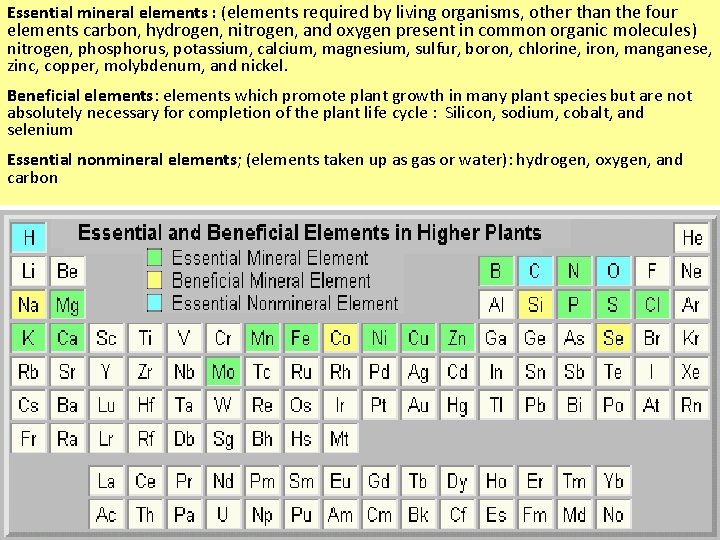
Essential mineral elements : (elements required by living organisms, other than the four elements carbon, hydrogen, nitrogen, and oxygen present in common organic molecules) nitrogen, phosphorus, potassium, calcium, magnesium, sulfur, boron, chlorine, iron, manganese, zinc, copper, molybdenum, and nickel. Beneficial elements: elements which promote plant growth in many plant species but are not absolutely necessary for completion of the plant life cycle : Silicon, sodium, cobalt, and selenium Essential nonmineral elements; (elements taken up as gas or water): hydrogen, oxygen, and carbon

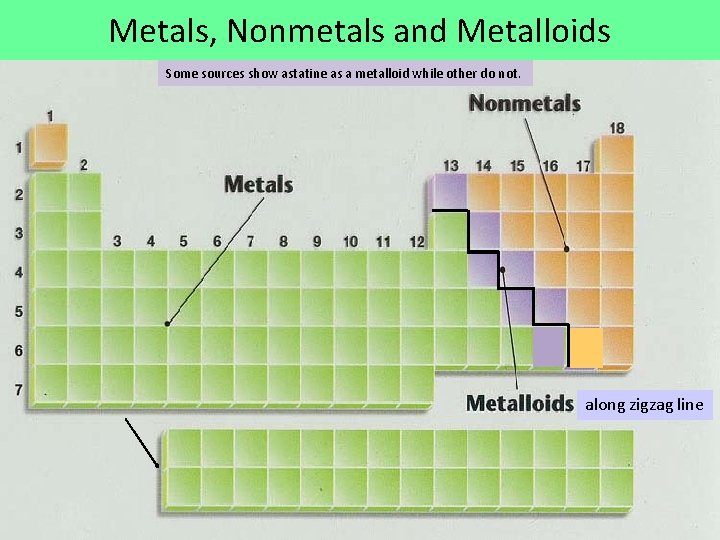
Metals, Nonmetals and Metalloids Some sources show astatine as a metalloid while other do not. along zigzag line

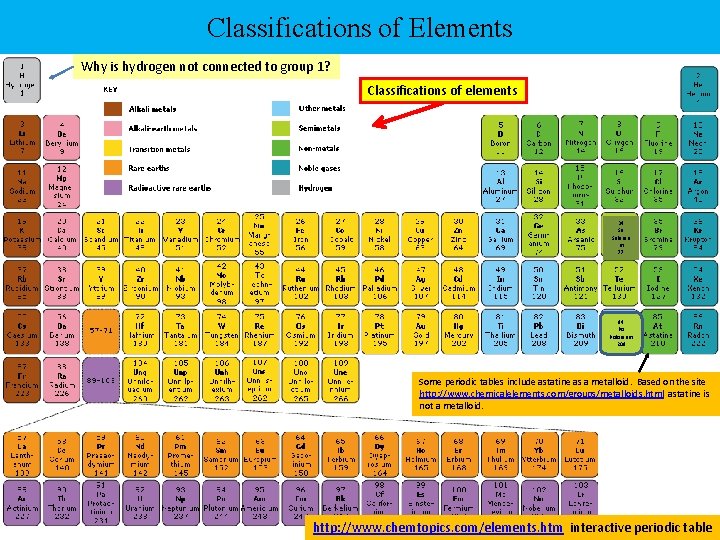
Classifications of Elements Why is hydrogen not connected to group 1? Classifications of elements 34 Se Seleniu m 77 84 Po Polonium 209 Some periodic tables include astatine as a metalloid. Based on the site http: //www. chemicalelements. com/groups/metalloids. html astatine is not a metalloid. http: //www. chemtopics. com/elements. htm interactive periodic table

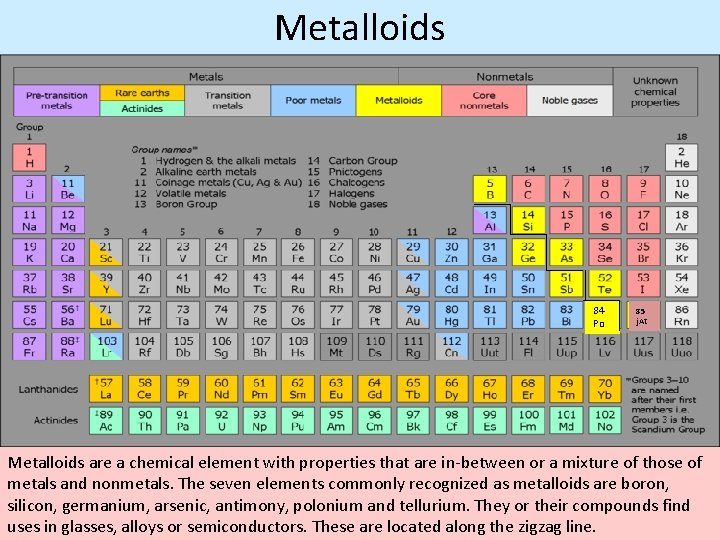
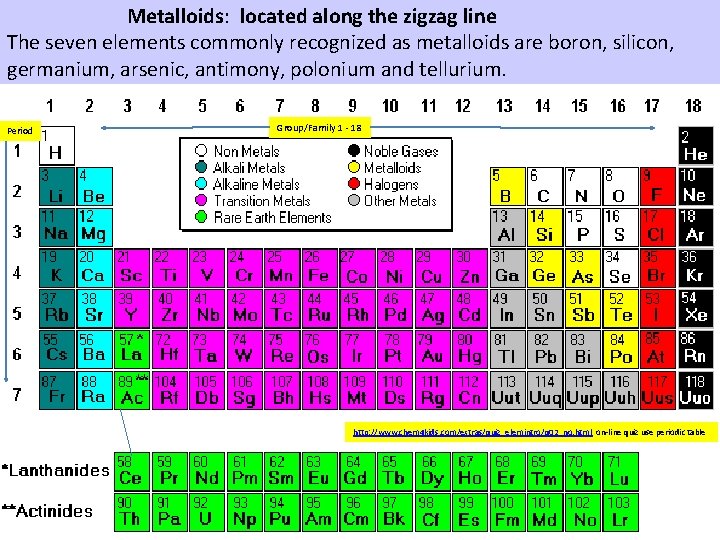
Metalloids 84 Po 85 j. At Metalloids are a chemical element with properties that are in between or a mixture of those of metals and nonmetals. The seven elements commonly recognized as metalloids are boron, silicon, germanium, arsenic, antimony, polonium and tellurium. They or their compounds find uses in glasses, alloys or semiconductors. These are located along the zigzag line.

Metalloids: located along the zigzag line The seven elements commonly recognized as metalloids are boron, silicon, germanium, arsenic, antimony, polonium and tellurium. Period Group/Family 1 18 http: //www. chem 4 kids. com/extras/quiz_elemintro/q 02_no. html on line quiz use periodic table


Metals Notice that the metalloids and nonmetals are not included. Compare this section of the periodic table to the complete periodic table of elements.

Metalloids & Nonmetals Metalloids Nonmetals Some sources show astatine as a metalloid while other do not.

Physical Properties: can be observed and measured without changing the kind of matter being studied; can be used to identify substances Melting Point: The temperature at which a solid can change to a liquid. The temperature at which a pure substance melts is unchanging under constant conditions. Boiling Point: The temperature at which a liquid boils. A substance changes from a liquid to a gas. Boiling temperature is unchanging under constant conditions for a given substance. Assessment Probe: “Floating Logs” Example: Man from Zambia Demo: Density Blocks Density: a property that describes the relationship between the mass of a material and its volume Substances that have higher densities contain more matter in a given volume. The density of a substance will stay constant/the same. Color: may be used to identify substances but not always

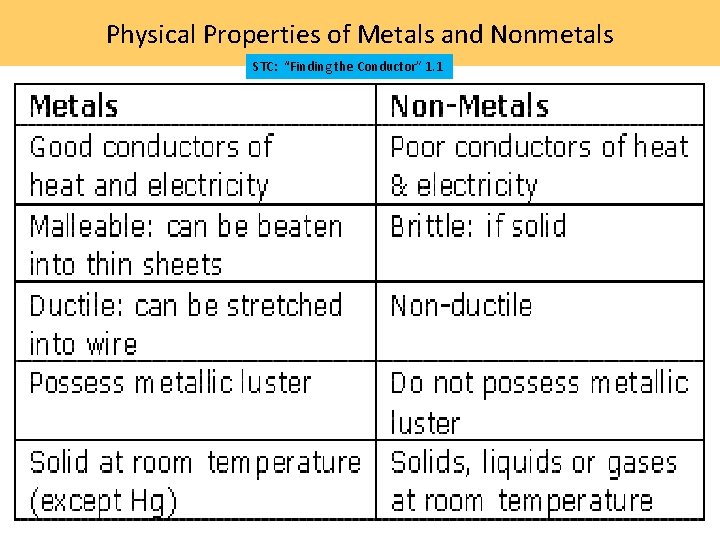
Physical Properties of Metals and Nonmetals STC: “Finding the Conductor” 1. 1

Physical Properties of Noble Gasses (Group/Family #18)

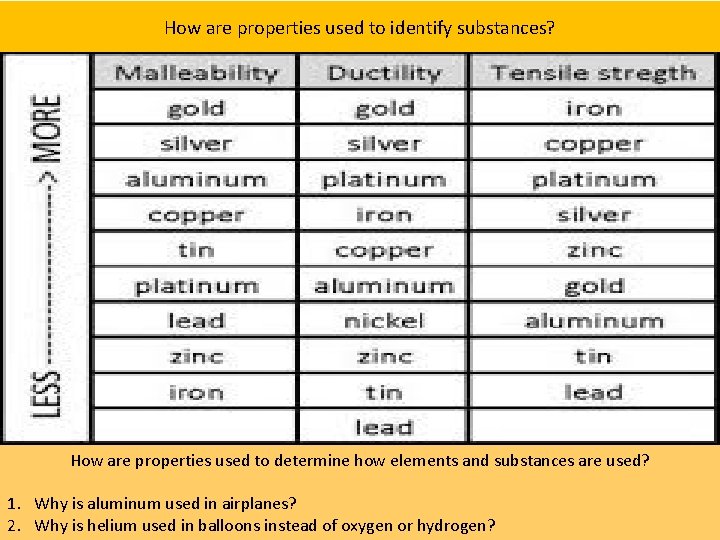
How are properties used to identify substances? H How are properties used to determine how elements and substances are used? 1. Why is aluminum used in airplanes? 2. Why is helium used in balloons instead of oxygen or hydrogen?

Chemical Property: any of a material's properties that becomes evident during a chemical reaction; that is, any quality that can be established only by changing a substance's chemical identity 1. Can be used to help identify a substance Example: Man from Zambia 2. Usually involves the substance’s ability to react or not react with another specific substance Examples Reacting with Oxygen (oxidation): The ability of a substance to burn is a chemical property that involves a substance reacting quickly with oxygen to produce light and heat. (i. e. iron rusts or apples turn brown). Reacting with Acids: The ability of a substance to react with an acid is a chemical property. Some metals react with various acids to form compounds. All metals do not react with all acids. Bases react with acids to form water and neutralize the acid.

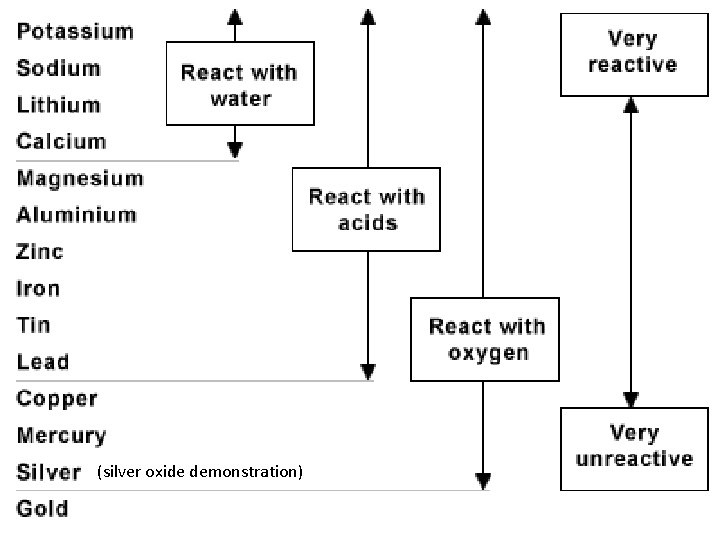
Reactivity: the tendency of a substance to undergo chemical reaction, either by itself or with other materials. ; reactivity is a chemical property of an element http: //www. youtube. com/watch? v=Jy 1 DC 6 Euqj 4 potassium reaction in water 20 sec. http: //www. youtube. com/watch? v=MTcgo 46 nx. NE sodium reaction in water 48 sec. These soft, silvery sodium chunks were cut with a knife and stored under oil. In air they turn white in seconds; exposed to water they generate hydrogen gas and explode in Flaming balls of molten sodium. http: //www. youtube. com/watch? v=1 b. JBue. GSC 9 M calcium reactivity with oxygen 27 sec.

(silver oxide demonstration)

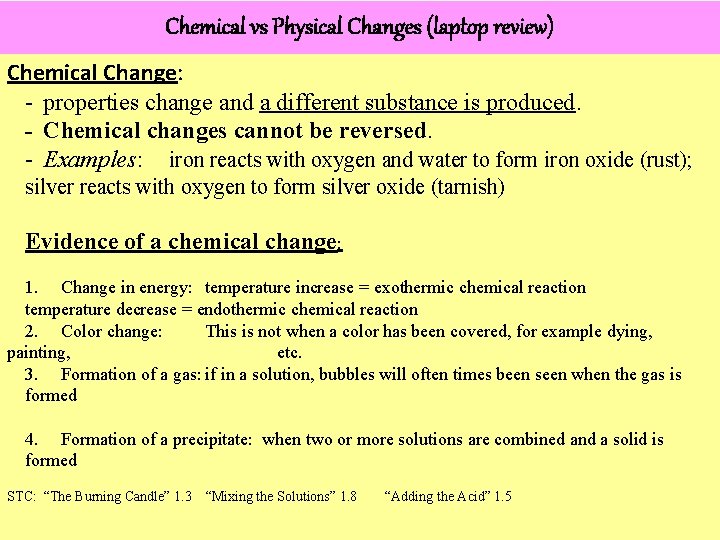
Physical & Chemical Properties Substance Physical Property Chemical Property Helium Less dense than air Nonflammable Wood Grainy texture Flammable/combustible Baking Soda White powder Reacts with vinegar to form bubbles Powdered Sugar White powder Does not react with vinegar Rubbing Alcohol Clear liquid Flammable/Combustible Red Food Coloring Red color Reacts with bleach and loses color Iron Malleable Reacts with oxygen (oxidation) to form iron oxide (rust) Tin Malleable Reacts with oxygen to form tin dioxide

Physical or Chemical Property… What do you think? 1. Shape 2. Density 3. Acidity (below 7 p. H) 4. Solubility 5. Basicity (above 7 p. H) 6. Combustibility 7. Odor 8. Melting point 9. Reactivity 10. Boiling point 11. Color Points to Consider If the property changes, is a new substance formed? If not, it is a physical property. If you still have the same substance after changing the property, it is a physical property.

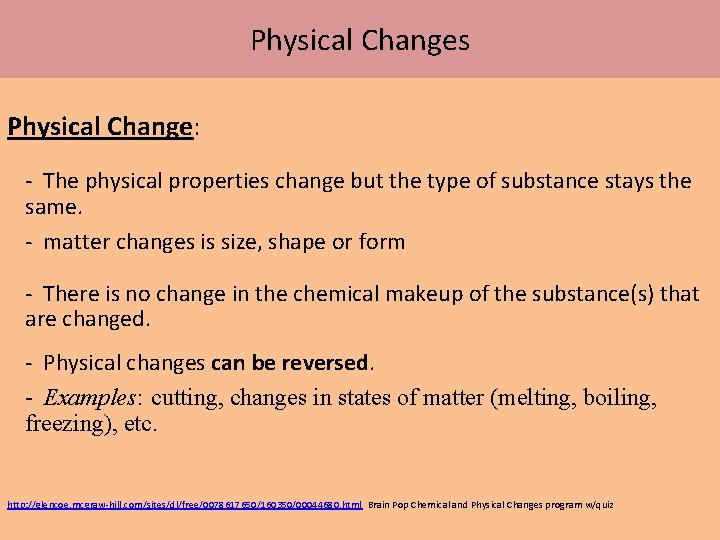
Chemical vs Physical Changes (laptop review) Chemical Change: - properties change and a different substance is produced. - Chemical changes cannot be reversed. - Examples: iron reacts with oxygen and water to form iron oxide (rust); silver reacts with oxygen to form silver oxide (tarnish) Evidence of a chemical change: 1. Change in energy: temperature increase = exothermic chemical reaction temperature decrease = endothermic chemical reaction 2. Color change: This is not when a color has been covered, for example dying, painting, etc. 3. Formation of a gas: if in a solution, bubbles will often times been seen when the gas is formed 4. Formation of a precipitate: when two or more solutions are combined and a solid is formed STC: “The Burning Candle” 1. 3 “Mixing the Solutions” 1. 8 “Adding the Acid” 1. 5

Physical Changes Physical Change: The physical properties change but the type of substance stays the same. matter changes is size, shape or form There is no change in the chemical makeup of the substance(s) that are changed. Physical changes can be reversed. - Examples: cutting, changes in states of matter (melting, boiling, freezing), etc. http: //glencoe. mcgraw hill. com/sites/dl/free/0078617650/160350/00044680. html Brain Pop Chemical and Physical Changes program w/quiz

Phase Change (mixing two or more substances) (dissolving a substance)

Sublimation: when a substance changes directly from a gas to a solid Examples: The forming of frost from water vapor When dry ice forms: Dry ice is frozen carbon dioxide. As it breaks down, it turns directly into carbon dioxide gas rather than a liquid http: //www. youtube. com/watch? v=8 t. HOVVg. Gkpk dry ice bubbles 2: 20; also shows properties of polar molecules water and detergent (surface tension) Solid air fresheners Apples brown (react with oxygen)

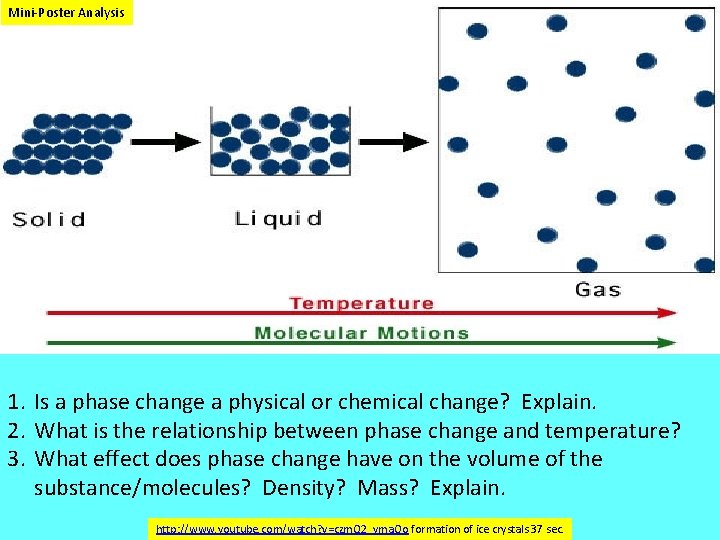
Mini Poster Analysis 1. Is a phase change a physical or chemical change? Explain. 2. What is the relationship between phase change and temperature? 3. What effect does phase change have on the volume of the substance/molecules? Density? Mass? Explain. http: //www. youtube. com/watch? v=czm. Q 2_yma. Oo formation of ice crystals 37 sec.

Chemical Changes Require Chemical Reactions

Chemical Reaction: when a substance (or a few substances) change into another substance. STC: “Reacting A Tablet” 1. 7 MRE Lab Endothermic Reactions = temperature decrease; Exothermic Reactions = Temperature increase A chemical change involves a physical change, and can include but is not limited to the following: Examples: change in color, texture, physical state, odor, production of a gas, formation of precipitate, a change in its solubility, burning, rusting, etc. Chemical changes do not change the mass, because according to the Law of Conservation of Mass/Matter, during a chemical reaction the mass of the reactants of the formula will always equal the mass of the products

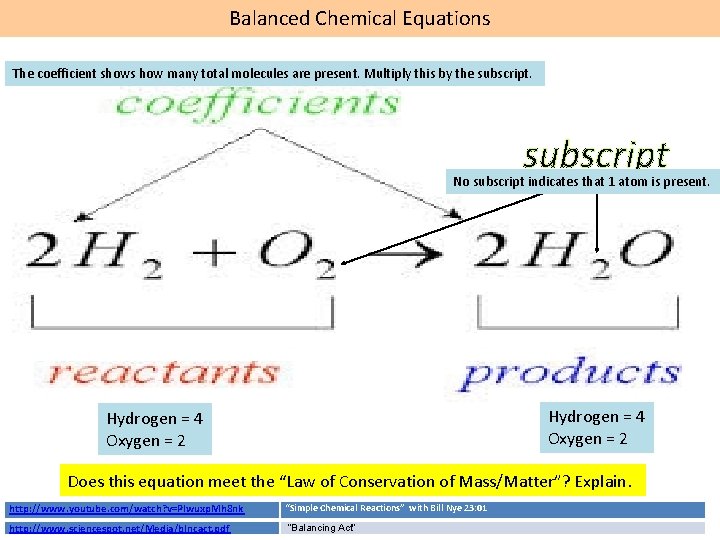
Chemical Reactions = Chemical Changes = New Substance(s) Formed Chemical reactions are shown in the form of chemical equations. Word equation: Sodium + Chlorine yields sodium chloride Chemical equation: 2 Na(s) + Cl 2 (g) 2 Na. Cl(s) Coefficient = # of molecules in the compound reactants products Subscript = # of atoms of the element 1. What is the mass of the reactants? 2. What is the mass of the products? 3. Does this reaction meet the “Law of Conservation of Mass/Matter”? Explain. Law of Conservation of Mass/Matter: “Nuts & Bolts of Law of Conservation of Mass” In a chemical equation, matter cannot be created or destroyed The mass of the reactants will equal the mass of the products. STC: “Mixing the Solutions” 1. 8 “Balancing Chemical Equations” practice

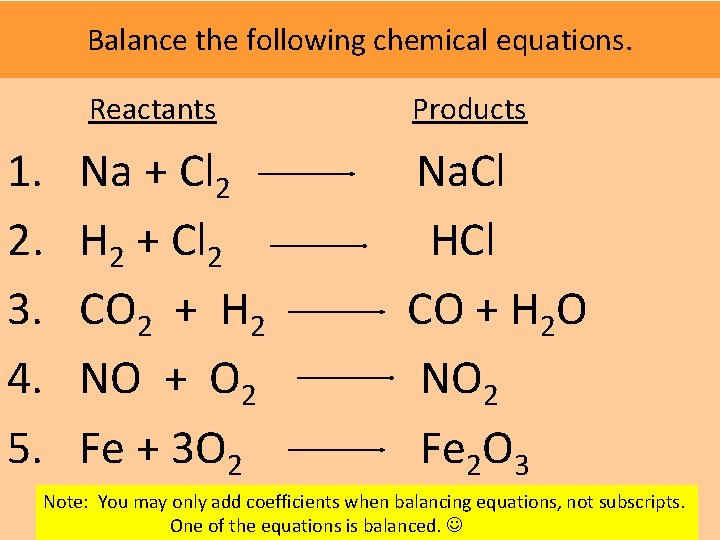
Balanced Chemical Equations The coefficient shows how many total molecules are present. Multiply this by the subscript No subscript indicates that 1 atom is present. Hydrogen = 4 Oxygen = 2 Does this equation meet the “Law of Conservation of Mass/Matter”? Explain. http: //www. youtube. com/watch? v=Plwuxp. Mh 8 nk “Simple Chemical Reactions” with Bill Nye 23: 01 http: //www. sciencespot. net/Media/blncact. pdf “Balancing Act”

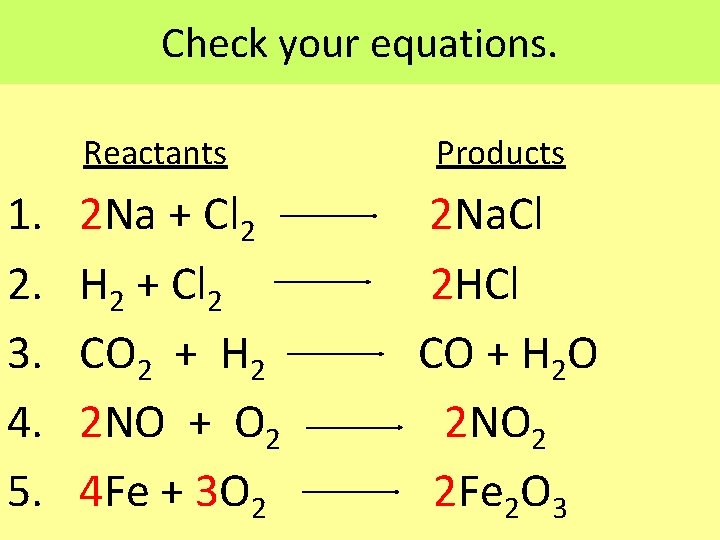
Balance the following chemical equations. Reactants Products 1. 2. 3. 4. 5. Na + Cl 2 Na. Cl H 2 + Cl 2 HCl CO 2 + H 2 CO + H 2 O NO + O 2 NO 2 Fe + 3 O 2 Fe 2 O 3 Note: You may only add coefficients when balancing equations, not subscripts. One of the equations is balanced.

Check your equations. Reactants Products 1. 2. 3. 4. 5. 2 Na + Cl 2 2 Na. Cl H 2 + Cl 2 2 HCl CO 2 + H 2 CO + H 2 O 2 NO + O 2 2 NO 2 4 Fe + 3 O 2 2 Fe 2 O 3

Balanced chemical equations support the law of conservation of mass/matter. Explain why. The chemical reaction for photosynthesis Reactants yield Products Reactants 6 carbon 24 oxygen 24 hydrogen Products 6 carbon 24 oxygen 24 hydrogen

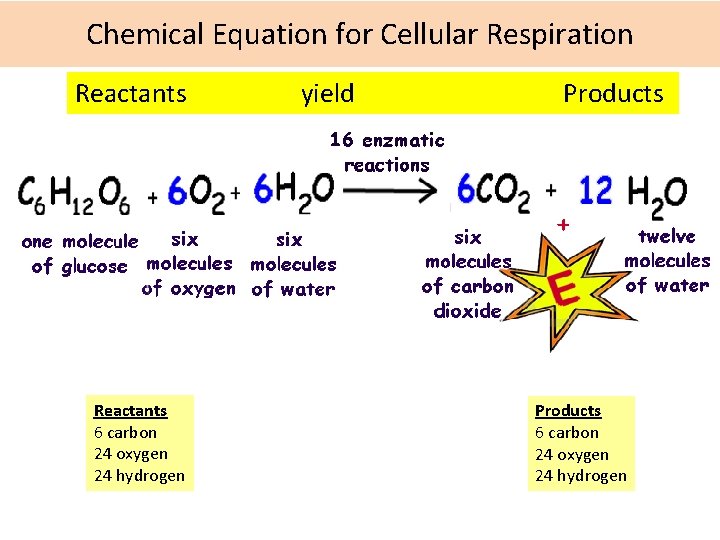
Chemical Equation for Cellular Respiration Reactants yield Products Reactants 6 carbon 24 oxygen 24 hydrogen Products 6 carbon 24 oxygen 24 hydrogen

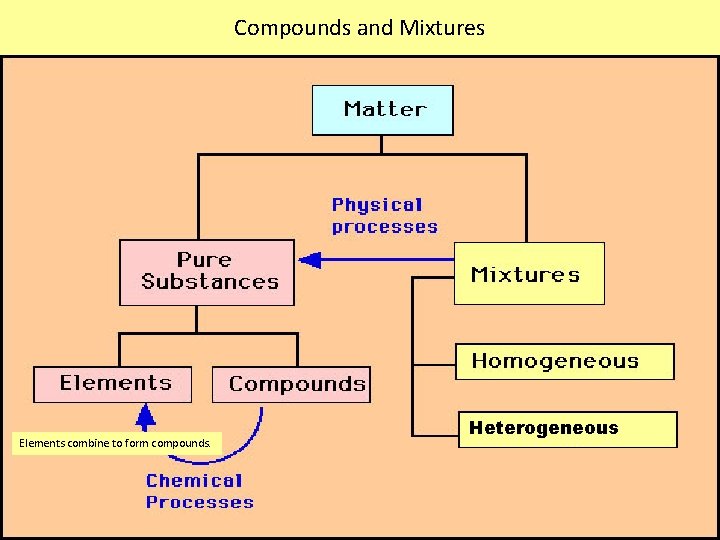
Compounds and Mixtures Elements combine to form compounds. Heterogeneous

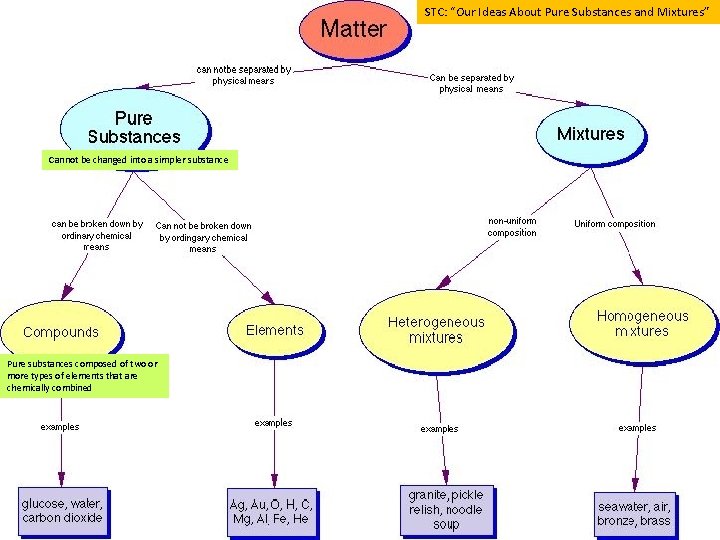
STC: “Our Ideas About Pure Substances and Mixtures” Cannot be changed into a simpler substance Pure substances composed of two or more types of elements that are chemically combined

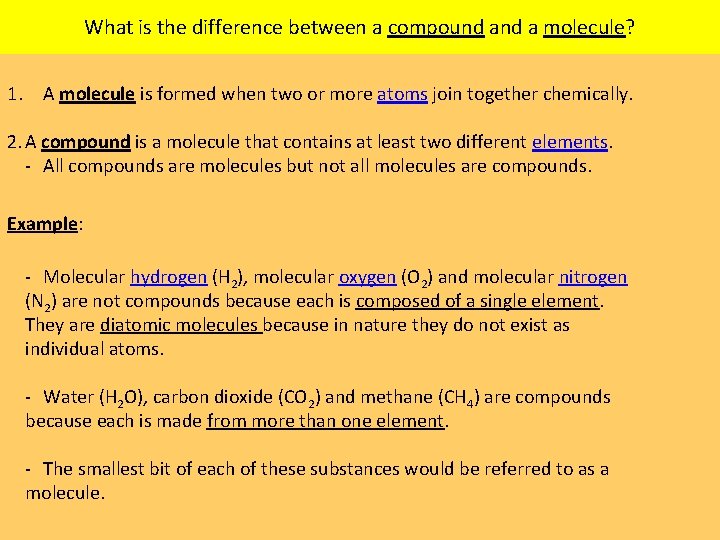
What is the difference between a compound a molecule? 1. A molecule is formed when two or more atoms join together chemically. 2. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. Example: Molecular hydrogen (H 2), molecular oxygen (O 2) and molecular nitrogen (N 2) are not compounds because each is composed of a single element. They are diatomic molecules because in nature they do not exist as individual atoms. Water (H 2 O), carbon dioxide (CO 2) and methane (CH 4) are compounds because each is made from more than one element. The smallest bit of each of these substances would be referred to as a molecule.

Compounds A compound is a type of matter that forms when two or more different elements combine chemically. Two or more atoms bonded together is a molecule of the substance that is formed. When atoms combine chemically, they do not retain their original properties. How do the following compounds provide evidence of this? In the compounds below, compare the properties of elements before and after chemical bonding has occurred. Examples: Chemical formulas for common substances H 2 O chemical formula for 1 water molecule Subscript: #of atoms present of the element to the left no # as with oxygen means there is 1 atom present C 12 H 22 O 11 Na. Cl O 2 Na. Cl. O HCl Na. Cl sucrose table salt oxygen (diatomic element) household bleach hydrochloric acid sodium chloride (table salt) Na. HCO 3 HC 2 H 3 O 2 H 2 O NH 3 CO 2 H 2 O 2 baking soda vinegar water ammonia carbon dioxide hydrogen peroxide Activity: Identification of Element & Compounds in Products “Analyzing Formulas” practice

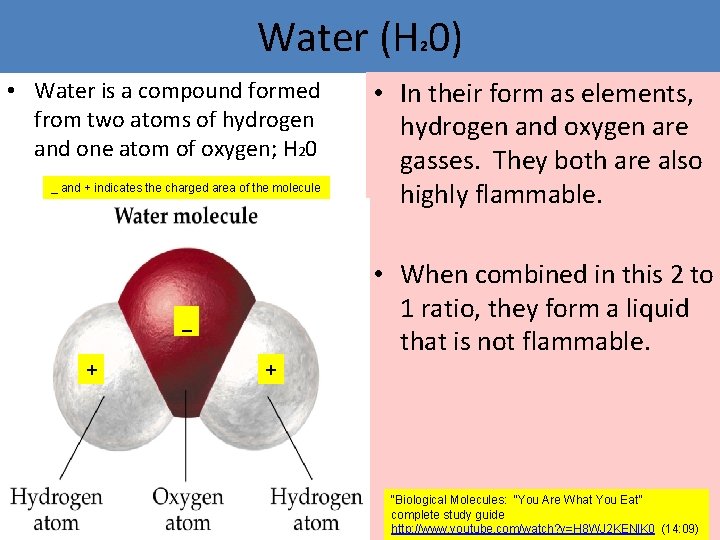
Water (H 0) 2 • Water is a compound formed from two atoms of hydrogen and one atom of oxygen; H 20 _ and + indicates the charged area of the molecule _ + • In their form as elements, hydrogen and oxygen are gasses. They both are also highly flammable. • When combined in this 2 to 1 ratio, they form a liquid that is not flammable. + “Biological Molecules: “You Are What You Eat” complete study guide http: //www. youtube. com/watch? v=H 8 WJ 2 KENl. K 0 (14: 09)

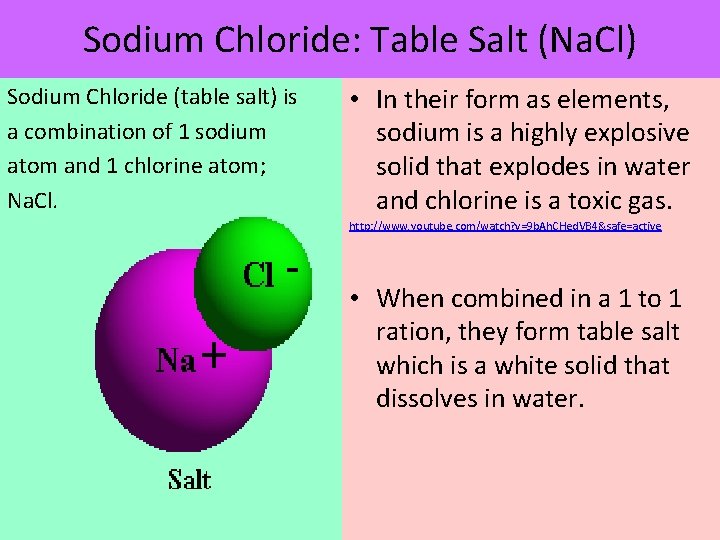
Sodium Chloride: Table Salt (Na. Cl) Sodium Chloride (table salt) is a combination of 1 sodium atom and 1 chlorine atom; Na. Cl. • In their form as elements, sodium is a highly explosive solid that explodes in water and chlorine is a toxic gas. http: //www. youtube. com/watch? v=9 b. Ah. CHed. VB 4&safe=active • When combined in a 1 to 1 ration, they form table salt which is a white solid that dissolves in water.

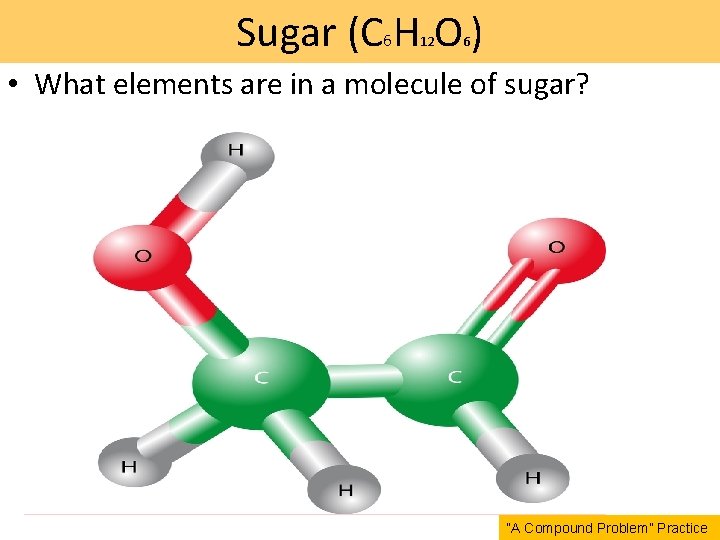
Sugar (C 6 H O ) 12 6 • What elements are in a molecule of sugar? “A Compound Problem” Practice

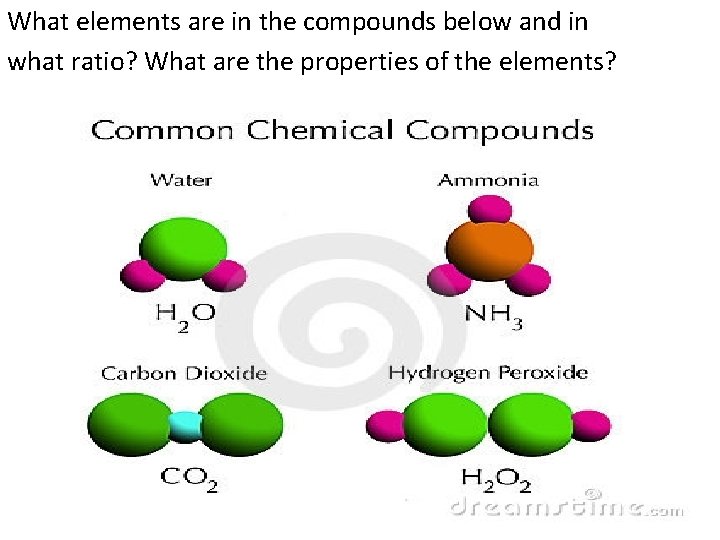
What elements are in the compounds below and in what ratio? What are the properties of the elements?

Glucose and Fructose (sugar) Molecules What elements are common is both of these compounds? How are they different?

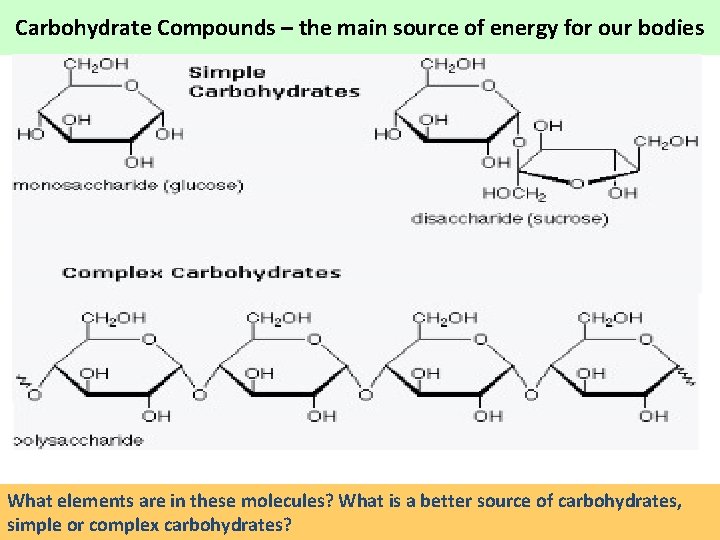
Carbohydrate Compounds – the main source of energy for our bodies What elements are in these molecules? What is a better source of carbohydrates, simple or complex carbohydrates?

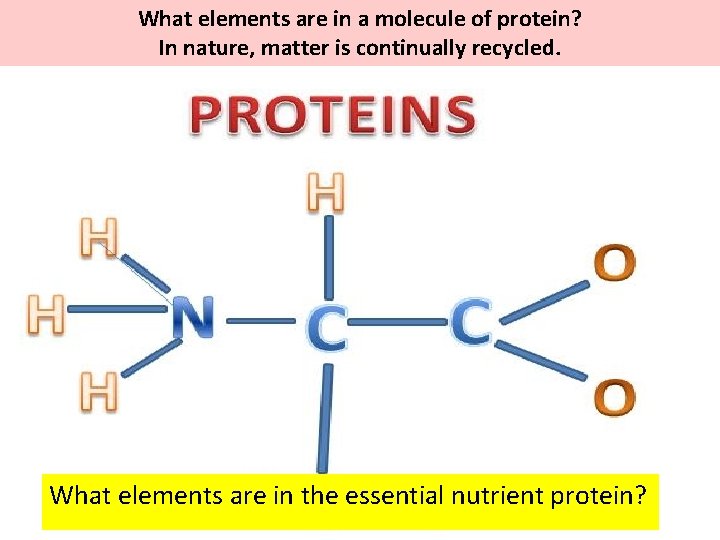
What elements are in a molecule of protein? In nature, matter is continually recycled. What elements are in the essential nutrient protein?

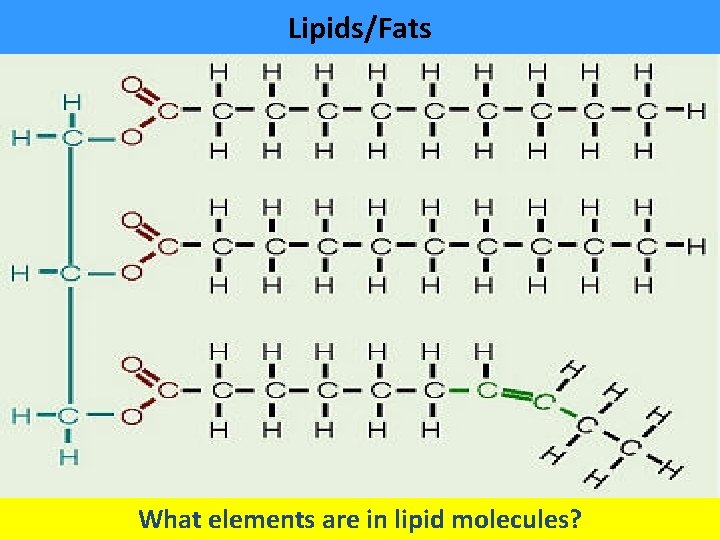
Lipids/Fats What elements are in lipid molecules?

Common Chemicals Common Name Chemical Formula white vinegar acetic acid CH 3 COOH + H 2 O nail polish remover acetone CH 3 COCH 3 ammonia ammonium hydroxide CH 3 + H 2 O boric acid H 3 BO 3 marble, limestone, chalk calcium carbonate Ca. CO 3 road salt calcium chloride Ca. Cl 2 garden lime calcium hydroxide Ca(OH)2 corn syrup glucose C 6 H 12 O 6 + H 2 O epsom salt magnesium sulfate Mg. SO 4 + 7 H 2 O moth balls naphthalene C 10 H 8 baking soda sodium bicarbonate Na. HCO 3 table salt sodium chloride Na. Cl cane sugar sucrose C 12 H 22 O 11 sulfuric acid H 2 SO 4

Review: Molecules & Compounds 1. A molecule is what you get when any atoms join together. 2. 2. A compound is what you get when atoms of two or more different elements join together. 3. All compounds are molecules, but not all molecules are compounds. Water is a molecule because it is made from atoms that have been chemically combined. It is also a compound because the atoms that Water is a molecule because it is made from atoms that have been chemically combined. It is also a compound because the atoms that make water are not all the same some are oxygen and some are hydrogen. Oxygen in the atmosphere is a molecule because it is made from two atoms of oxygen. It is not a compound because it is made from atoms of only one element oxygen. This type of molecule is called a diatomic Oxygen in the atmosphere is a molecule because it is made from two molecule, a molecule made from two atoms of the same type. atoms of oxygen. It is not a compound because it is made from atoms of only one element oxygen. This type of molecule is called a diatomic molecule, a molecule made from two atoms of the same type.

The Difference Between Compounds and Mixtures Mixture A physical blend of two or more substances that are NOT chemically combined. Compound A substance that contains two or more elements CHEMICALLY combined in a fixed proportion. The elements do not retain their original properties. Compound is chemically combined, mixture is not chemically combined. *Cake would be a compound because all of the ingredients are together to make batter you cannot see the individual components (eggs, flour, sugar, etc. ) *Trail mix would be a mixture because you can see every individual item (M&M, peanuts, raisins, pizza, etc. ) “Introduction to Matter” Mini poster examples

STC lab activities: “Comparing the Two Mixtures” 1. 6 “Filtering a Mixture” 1. 2 “Separating a Mixture” 1. 4 Mixture Ways to separate mixtures: Filtration (separates large particles from small) Evaporation (use boiling point) Centrifuge (use density) Magnet Sifting tin + copper = pewter copper + tin = bronze (more) (less) (more) (less) Steel is an alloy of iron, with carbon being the primary alloying element. The air you breathe is made up of about 21% oxygen, 78% nitrogen, and small amounts of other gases like argon, carbon dioxide and methane.

Homogenous or Heterogeneous Mixtures… Do you know? #1 M & M #2 Salad #3 Kool Aid drink #4 Fruit Loops #8 Hot Chocolate #5 Milk #6 Cake Batter #7 Chocolate Chip Cookie #9 Trail Mix #10 Tap Water #11 Smoke #12 Clear Seawater
