Think Pair Share What can you tell me








- Slides: 11

Think, Pair, Share – What can you tell me about acids and bases? – Can you think of some acids and bases that you might find at home? – Are all acids harmful? – What foods that we eat or drink are bases? Can you tell the difference by taste?

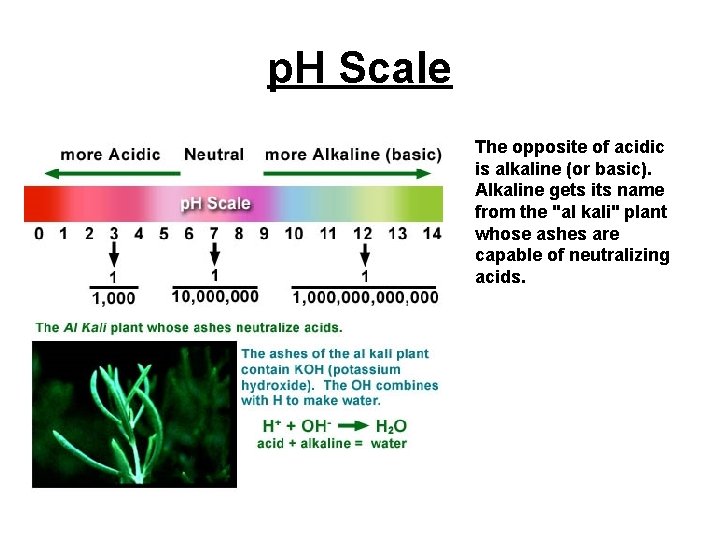
p. H Basics • The p. H scale is a measure of hydrogen ion concentrations. On the p. H scale, high concentrations of hydrogen ions are denoted by low p. H and low concentrations of hydrogen ions are denoted by high p. H.

p. H – “Potential for Hydrogen” • Red cabbage extract changes to many colors depending on acidity. Acidity is measured on a p. H scale. p. H stands for "potential for Hydrogen. " • Acidity is caused by hydrogen atoms that have lost their electrons and are roaming free in water [H+]. The scale goes from 0 to 14.

More Info on Atoms, Molecules , & Ions • http: //www. miamisci. org/ph/hoh. html • Please go to the website above for more information on atoms, molecules, and ions.

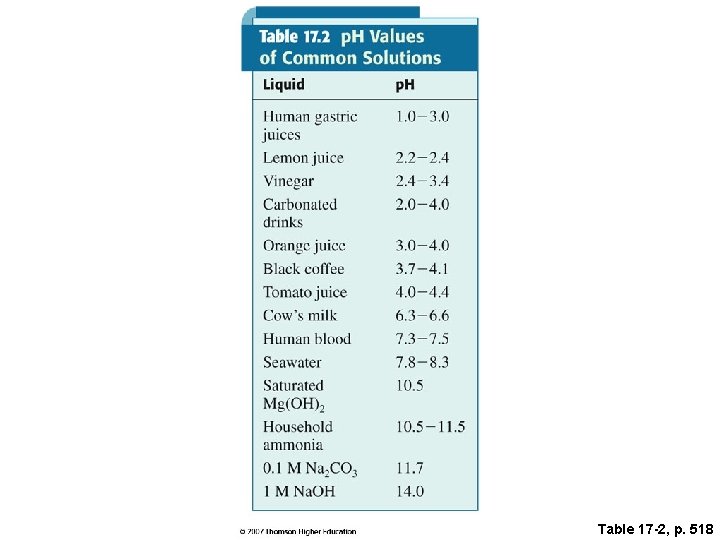
Table 17 -2, p. 518

p. H Scale The opposite of acidic is alkaline (or basic). Alkaline gets its name from the "al kali" plant whose ashes are capable of neutralizing acids.

Cabbage Juice • There are substances which have the property of changing their color when they come in contact with an acidic or basic environment. These substances are called p. H indicators. • Red cabbage juice is a natural p. H indicator.

Cabbage Juice • Cabbage juice turns bright pink in the presence of an acid. • Cabbage juice turns blue/green/yellow in the presence of a base.

Grape Juice • Grape juice turns deep red in the presence of an acid. • Grape juice turns blue in the presence of a base. • Grape juice is a natural p. H indicator.

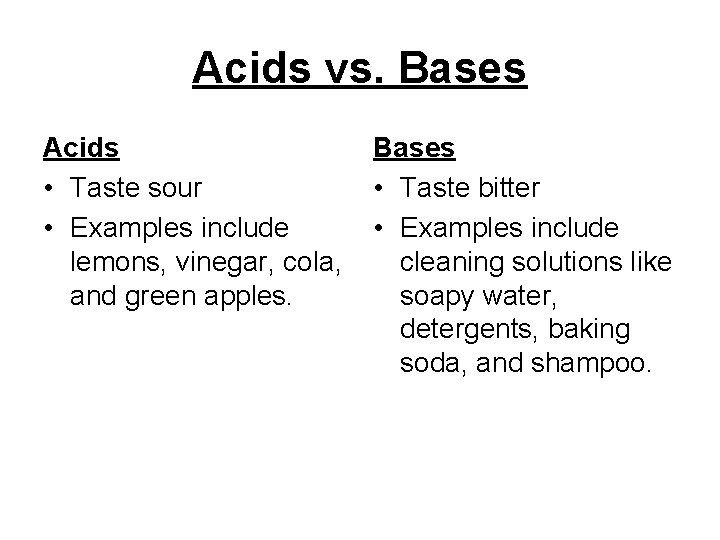
Acids vs. Bases Acids • Taste sour • Examples include lemons, vinegar, cola, and green apples. Bases • Taste bitter • Examples include cleaning solutions like soapy water, detergents, baking soda, and shampoo.

Extra Info for the Test • Distilled water is a neutral. • High amounts of hydrogen ions mean a low p. H value. This is an acid. • Low amounts of hydrogen ions mean a high p. H value. This is a base. • Examples of Acids – lemon juice, orange juice, vinegar, colas, and green apples • Examples of Bases – soaps, detergents, baking soda, shampoo, borax, limewater, and ammonia.
 If you think you can you can poem
If you think you can you can poem Tell me what you eat and i shall tell you what you are
Tell me what you eat and i shall tell you what you are Cone of learning
Cone of learning Kagan activities
Kagan activities Pair and share activity
Pair and share activity Study jams seasons
Study jams seasons Think pair share poster
Think pair share poster Think pair share cooperative learning
Think pair share cooperative learning What are think-pair-share debates?
What are think-pair-share debates? Share and discuss
Share and discuss Think pair share advantages and disadvantages
Think pair share advantages and disadvantages Darlene redmond
Darlene redmond





















