Things you have to have memorized Common polyatomic













- Slides: 16

Things you have to have memorized

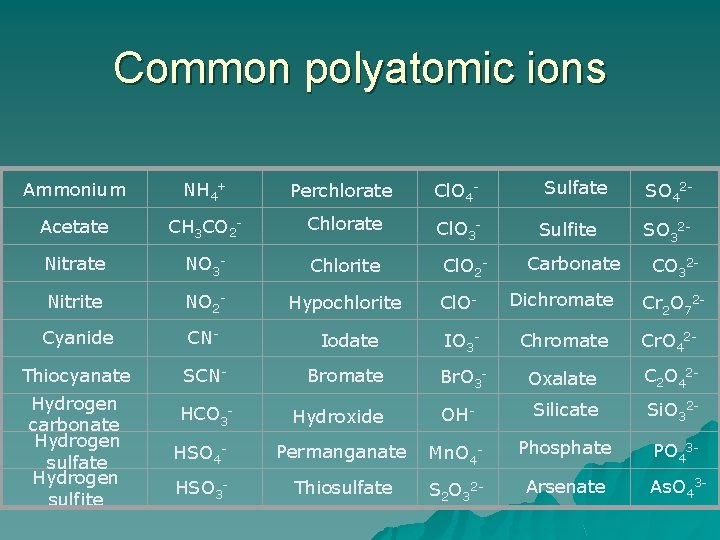
Common polyatomic ions Ammonium NH 4+ Perchlorate Cl. O 4 - Sulfate SO 42 - Acetate CH 3 CO 2 - Chlorate Cl. O 3 - Sulfite SO 32 - Nitrate NO 3 - Chlorite Nitrite NO 2 - Hypochlorite Cl. O- Dichromate Cr 2 O 72 - Cyanide CN- Iodate IO 3 - Chromate Cr. O 42 - Thiocyanate SCN- Bromate Br. O 3 - Oxalate C 2 O 42 - Hydroxide OH- Silicate Si. O 32 - HSO 4 - Permanganate Mn. O 4 - Phosphate PO 43 - HSO 3 - Thiosulfate S 2 O 32 - Arsenate As. O 43 - Hydrogen carbonate Hydrogen sulfite HCO 3 - Cl. O 2 - Carbonate CO 32 -

Strong Acids/Bases u Strong acids and bases completely dissociate in water. u For any net ionic equation, they will normally be H+ (H 3 O+) or OHu Generally, the conjugate acid or base is a spectator ion

Strong acids Acid formula Acid Formula Hydrochloric acid Hydrobromic acid Hydriodic acid HCl Sulfuric Acid Nitric Acid H 2 SO 4 Perchloric Acid HCl. O 4 HBr HI HNO 3 All Halogens except Fluorine. Get the F out. All oxyanions where there are 2 or more oxygen atoms than hydrogen

Strong Bases u All group 1 and 2 elements with hydroxide form strong bases. u However, most of them are not very soluble. u Although solubility and strength of bases are 2 different equilibrium equations.

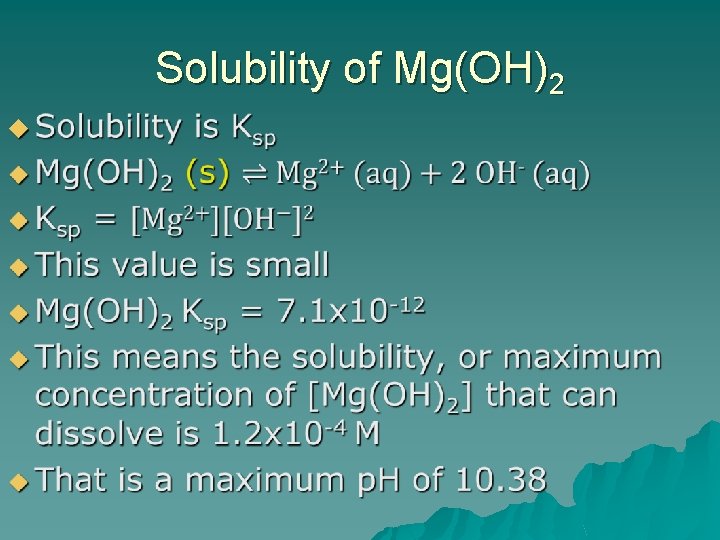
Solubility of Mg(OH)2 u

Base Strength of Mg(OH)2 u

Strong Bases that are somewhat soluble and not incredibly expensive Name Formula these make a lightning bolt on the periodic table! Name Formula Sodium Na. OH Hydroxide Calcium Ca(OH)2 Hydroxide Potassium KOH Hydroxide Strontium Sr(OH)2 Hydroxide Barium Ba(OH)2 Hydroxide

Mercury I u Mercury I is a polycation. u It is found as Hg 22+ u There are two mercury atoms covalently bonded together to make this ion. u Mercury I fluoride is u Hg 2 F 2

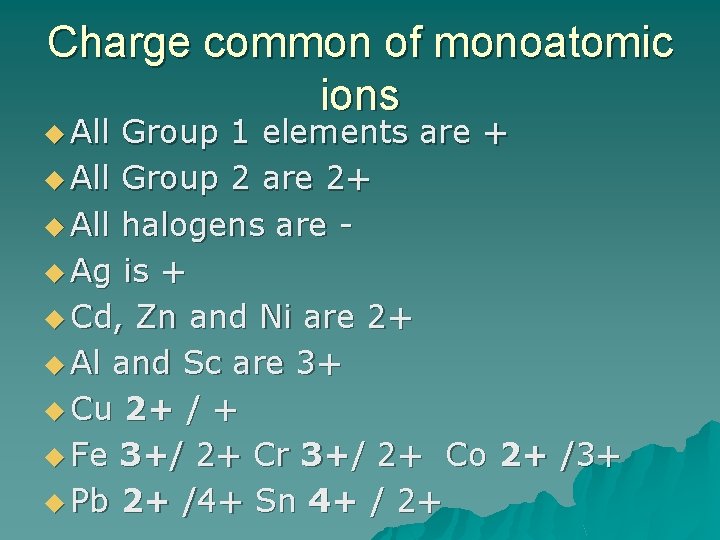
Charge common of monoatomic ions u All Group 1 elements are + u All Group 2 are 2+ u All halogens are u Ag is + u Cd, Zn and Ni are 2+ u Al and Sc are 3+ u Cu 2+ / + u Fe 3+/ 2+ Cr 3+/ 2+ Co 2+ /3+ u Pb 2+ /4+ Sn 4+ / 2+


Solubility Rules u Acids are soluble. u Compounds of: alkali metals, ammonium, nitrate, chlorate are soluble. u SNAP sodium nitrates ammonium and potassium are soluble u Halogens and silver, mercury or lead are insoluble (Ag. Cl is the most common)

u Most Solutions solutions are clear. u Copper solutions are blue. u Nickel solutions are green u Iron III is yellow to orange. u (Iron II is light blue) u Permanganate is purple u Chromate is yellow u Dichromate is orange u Metal coordination complexes are a variety of colors.

Precipitates/metal salts u Most precipitates are white u Copper precipitates are blue u Iron III are orange

Gases u H 2, He, NH 3, CH 4 float. Everything else sinks in regular air. u All noble gases are nonreactive (will put out a flame. u CO 2 and N 2 also put out a flame. u O 2 reignites a smoldering splint. u H 2 “pops” in a flame u CH 4 burns with O 2

Not for AP Metal Ion Flame Tests Ion Color Li+ Na+ K+ Ca 2+ Sr 2+ Ba 2+ Cu 2+ Fe 3+ Red Yellow Pink/Purple Orange/Red Green Blue/Green Gold