Things to remember Electrons have a negative charge

Things to remember! • Electrons have a negative charge • Protons have a positive charge • If an atom loses an electron it will become more positive because it has more protons. • If an atom gains an electron it will become more negative because it has more electrons.
Ions • Atoms had the same number of protons and electrons. • It has no charge because the positive and negative charges are balanced. • An atom can become an ion if it loses or gains an electron. • An ion has a charge, either becomes more positive or negative.
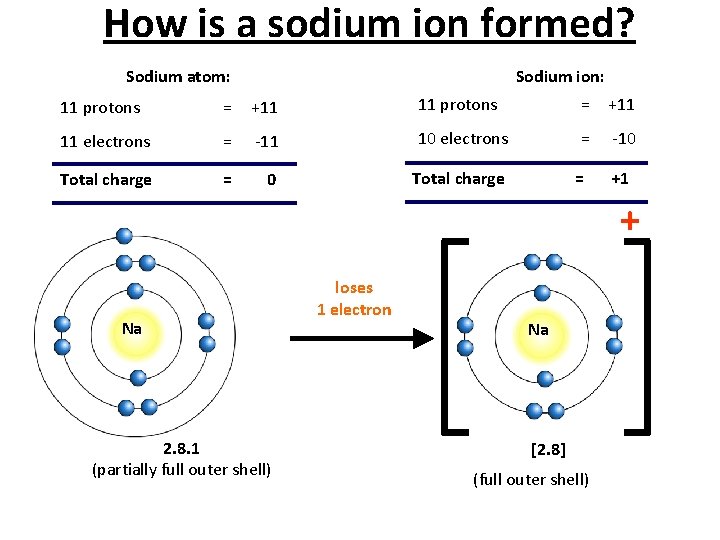
How is a sodium ion formed? Sodium atom: Sodium ion: 11 protons = +11 11 electrons = -11 10 electrons = -10 Total charge = +1 + Na 2. 8. 1 (partially full outer shell) loses 1 electron Na [2. 8] (full outer shell)
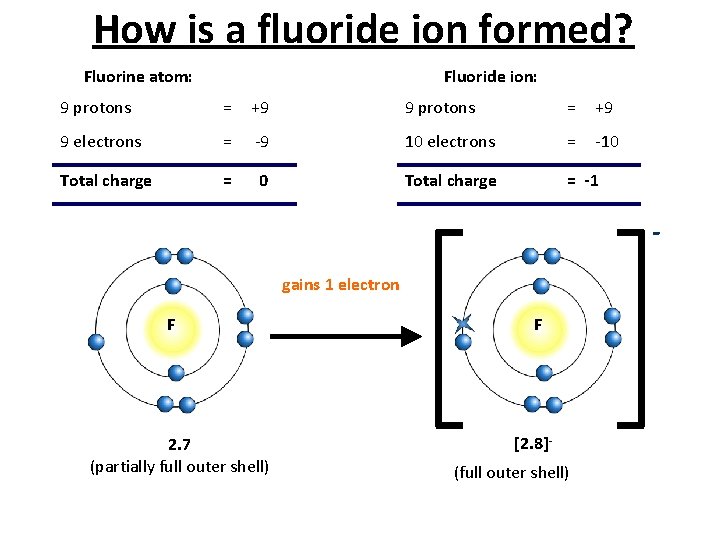
How is a fluoride ion formed? Fluorine atom: Fluoride ion: 9 protons = +9 9 electrons = -9 10 electrons = -10 Total charge = -1 gains 1 electron F 2. 7 (partially full outer shell) F [2. 8](full outer shell)

Cations • Metal atoms lose electrons to form positively charged ions called cations. For example, sodium atoms Na become sodium ions Na+. The + sign shows that a sodium ion carries a single positive charge. 22/01/2022

Anions • Non-metal atoms gain electrons to form negatively charged ions called anions. For example, chlorine atoms Cl become chloride ions Cl-. The – sign shows that a chloride ion carries a single negative charge. • Notice that the end of the name changes to –ide, when non-metal atoms form ions. 22/01/2022
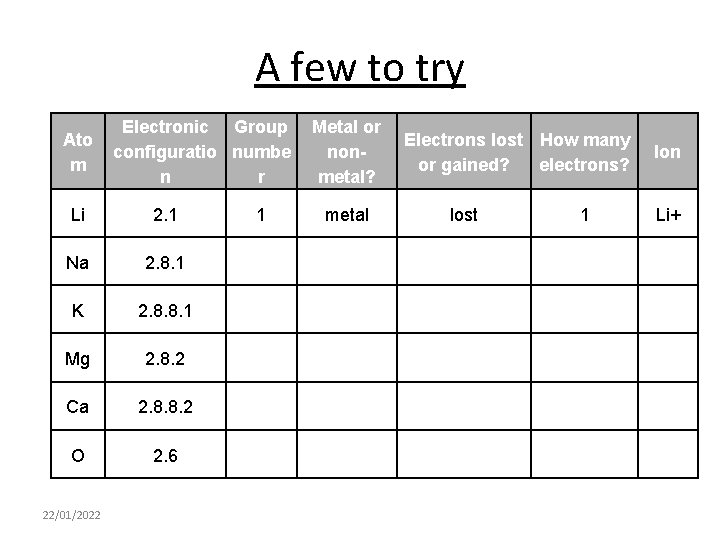
A few to try Ato m Electronic Group configuratio numbe n r Li 2. 1 Na 2. 8. 1 K 2. 8. 8. 1 Mg 2. 8. 2 Ca 2. 8. 8. 2 O 2. 6 22/01/2022 1 Metal or nonmetal? metal Electrons lost How many or gained? electrons? lost 1 Ion Li+
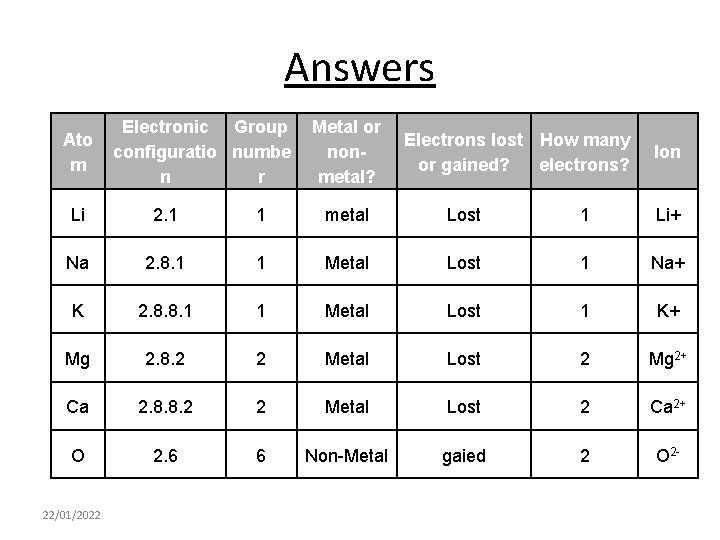
Answers Ato m Electronic Group configuratio numbe n r Metal or nonmetal? Electrons lost How many or gained? electrons? Ion Li 2. 1 1 metal Lost 1 Li+ Na 2. 8. 1 1 Metal Lost 1 Na+ K 2. 8. 8. 1 1 Metal Lost 1 K+ Mg 2. 8. 2 2 Metal Lost 2 Mg 2+ Ca 2. 8. 8. 2 2 Metal Lost 2 Ca 2+ O 2. 6 6 Non-Metal gaied 2 O 2 - 22/01/2022
- Slides: 8