Thermogravimetry which is one form of volatilization gravimetry

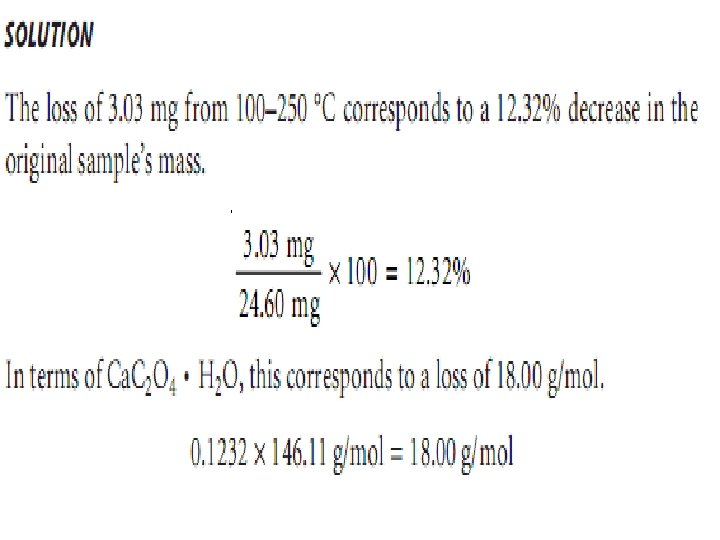

- Slides: 13

Thermogravimetry, which is one form of volatilization gravimetry, the sample’s mass is continuously monitored while the applied temperature is slowly increased. This requirement is rarely a problem for organic compounds in which volatilization is usually accomplished by combustion and the products are gases such as CO 2, H 2 O, and N.

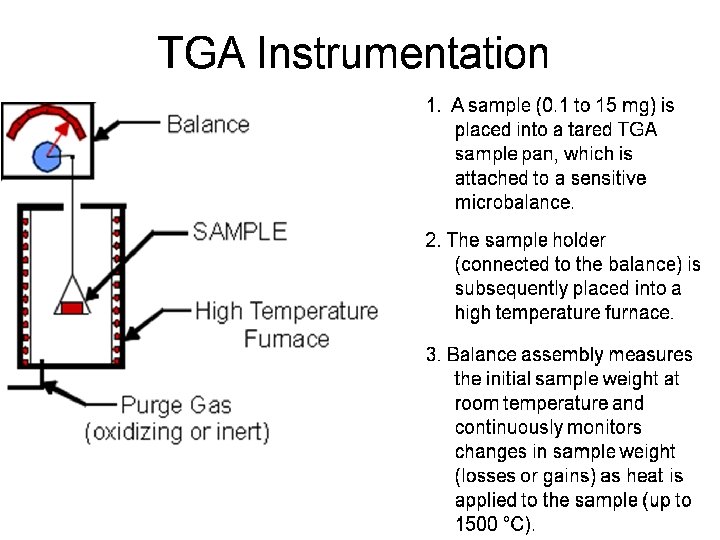
Thermal Gravimetric Analysis (TGA) gave information about: - • Evaluate thermal decomposition and stability of materials — Polymers, resins, rubbers, explosives • Information on bulk composition of compounds — Thermal oxidation, heat resistance — Residual water or solvents — Compositional analysis — Ash content in a sample — Quantity of inorganic filler in a polymer


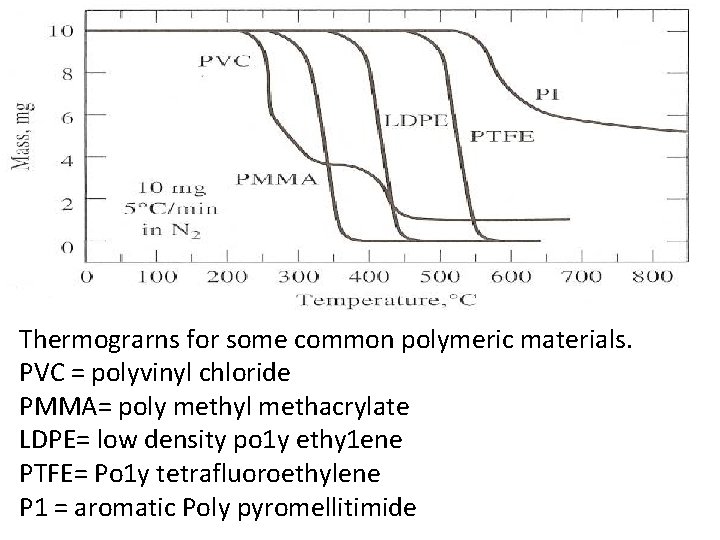
Thermograrns for some common polymeric materials. PVC = polyvinyl chloride PMMA= poly methyl methacrylate LDPE= low density po 1 y ethy 1 ene PTFE= Po 1 y tetrafluoroethylene P 1 = aromatic Poly pyromellitimide

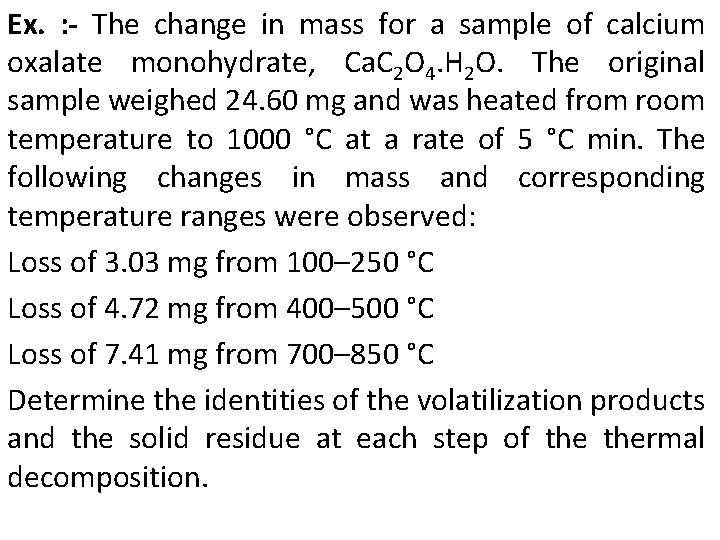
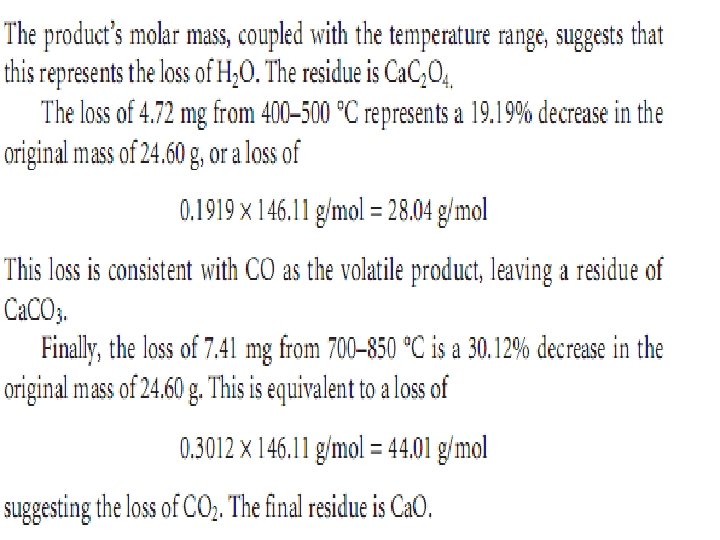
Ex. : - The change in mass for a sample of calcium oxalate monohydrate, Ca. C 2 O 4. H 2 O. The original sample weighed 24. 60 mg and was heated from room temperature to 1000 °C at a rate of 5 °C min. The following changes in mass and corresponding temperature ranges were observed: Loss of 3. 03 mg from 100– 250 °C Loss of 4. 72 mg from 400– 500 °C Loss of 7. 41 mg from 700– 850 °C Determine the identities of the volatilization products and the solid residue at each step of thermal decomposition.
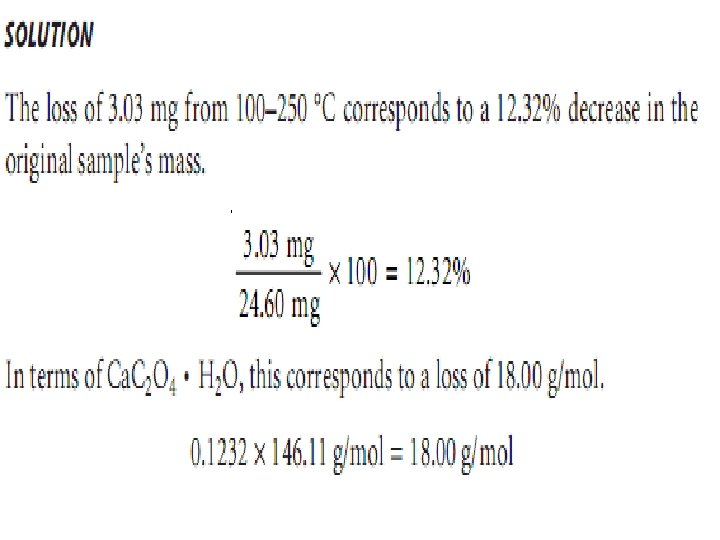


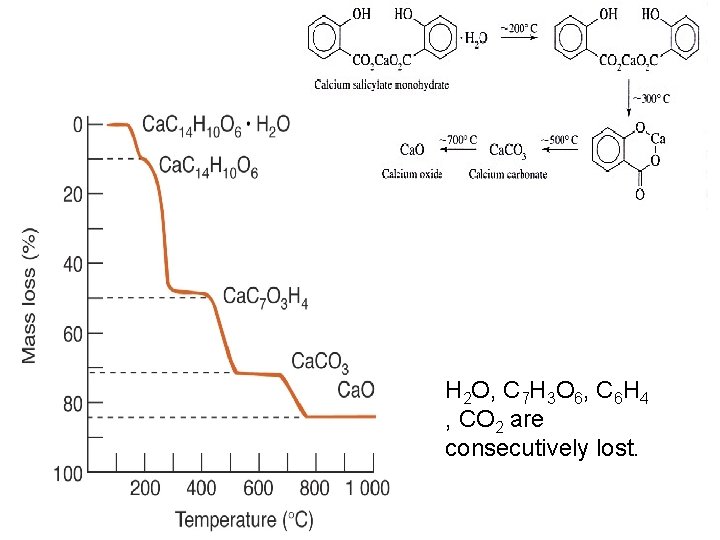
H 2 O, C 7 H 3 O 6, C 6 H 4 , CO 2 are consecutively lost.

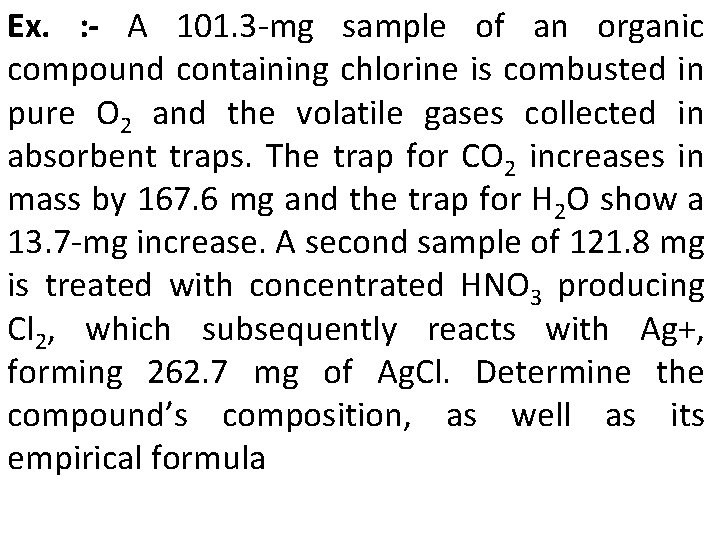
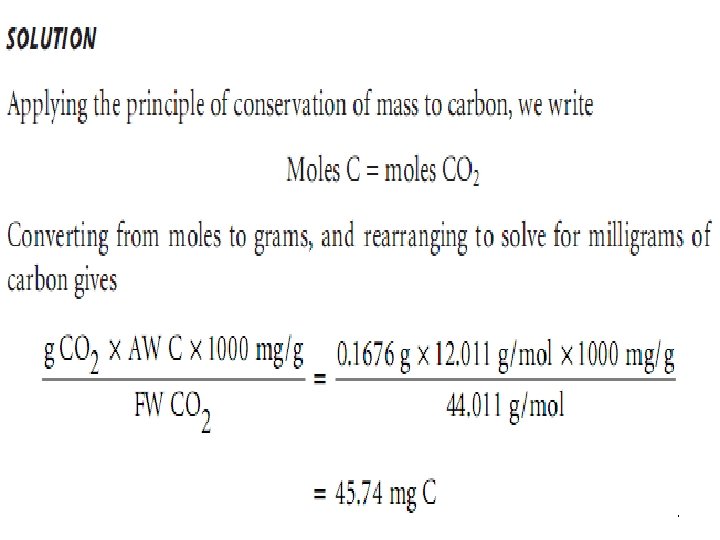
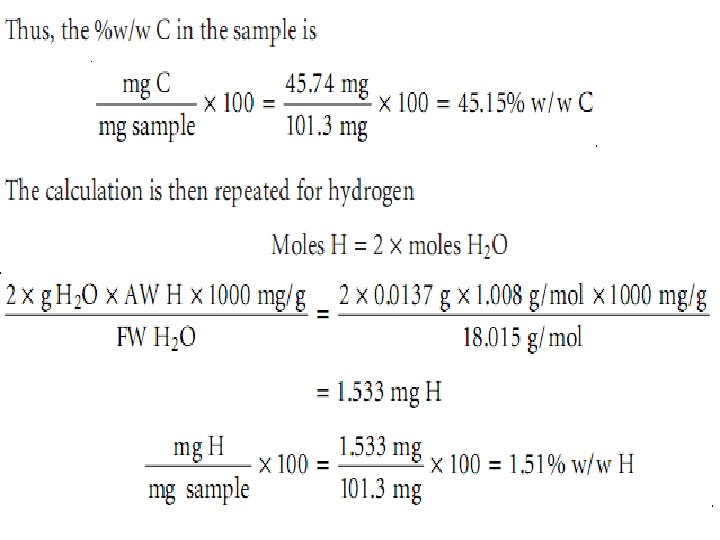
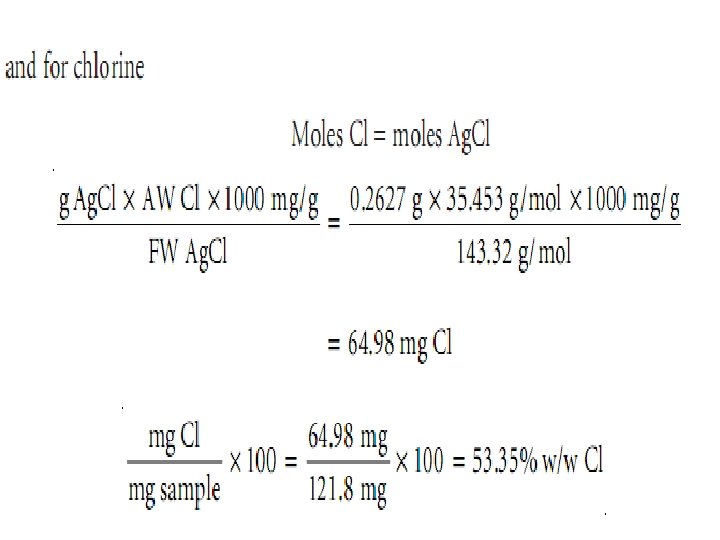
Ex. : - A 101. 3 -mg sample of an organic compound containing chlorine is combusted in pure O 2 and the volatile gases collected in absorbent traps. The trap for CO 2 increases in mass by 167. 6 mg and the trap for H 2 O show a 13. 7 -mg increase. A second sample of 121. 8 mg is treated with concentrated HNO 3 producing Cl 2, which subsequently reacts with Ag+, forming 262. 7 mg of Ag. Cl. Determine the compound’s composition, as well as its empirical formula




Exercise 1 Naturally occurring gypsum is a hydrated salt of calcium sulphate(Ca. SO 4. XH 2 O). A 67. 5 gm sample of gypsum is heated in a crucible until a constant mass is obtained. The mass of anhydrous salt (Ca. SO 4) was found to be 53. 4 gm, calculate : a- The percent by mass of water in the gypsum sample (hydrated salt). b-What is the formula of gypsum ? Exercise 2 A 695 gm of Cu. SO 4. XH 2 O is heated in a crucible until a constant mass is obtained and the mass of the anhydrous salt (Cu. SO 4) was found to be 444 gm, if you know that the F. W. of H 2 O = 18 gm/mol and the F. W. of anhydrous salt Cu. SO 4 = 159. 54 gm/mol, calculate a- The percent by mass of water in the hydrated salt. b-What is the formula of hydrated salt ?