Thermodynamics Thermo heat Dynamics movement Thermodynamics Our objectives
Thermodynamics Thermo= heat Dynamics= movement

Thermodynamics Our objectives are to: n define temperature, heat, and specific heat n describe three methods of heat transfer n discuss everyday examples to illustrate these concepts
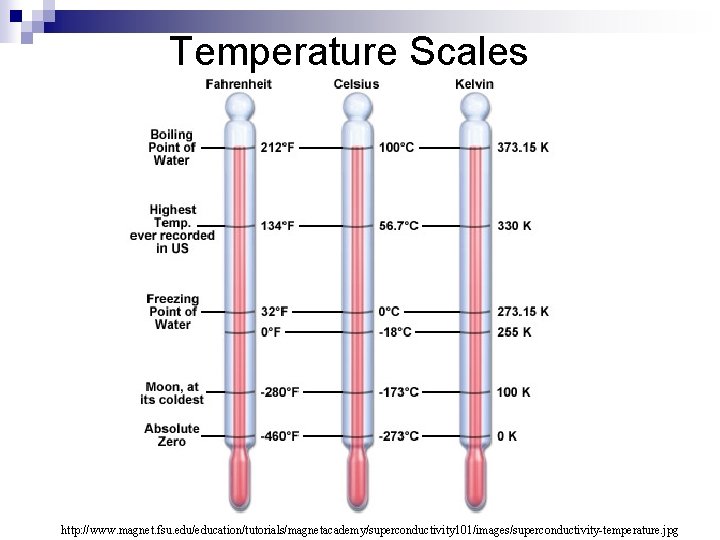
Temperature Scales http: //www. magnet. fsu. edu/education/tutorials/magnetacademy/superconductivity 101/images/superconductivity-temperature. jpg
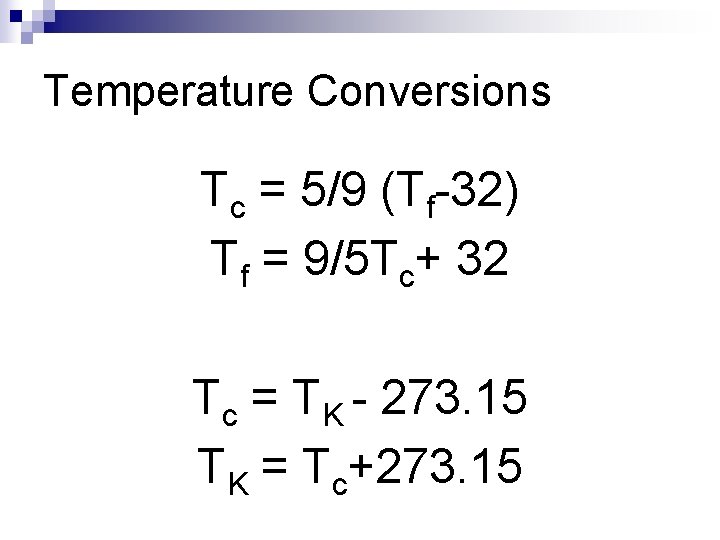
Temperature Conversions Tc = 5/9 (Tf-32) Tf = 9/5 Tc+ 32 Tc = TK - 273. 15 TK = Tc+273. 15

Applying the formulas (add this to your lab sheet) n Calculate the water temperature for each beaker in Kelvin
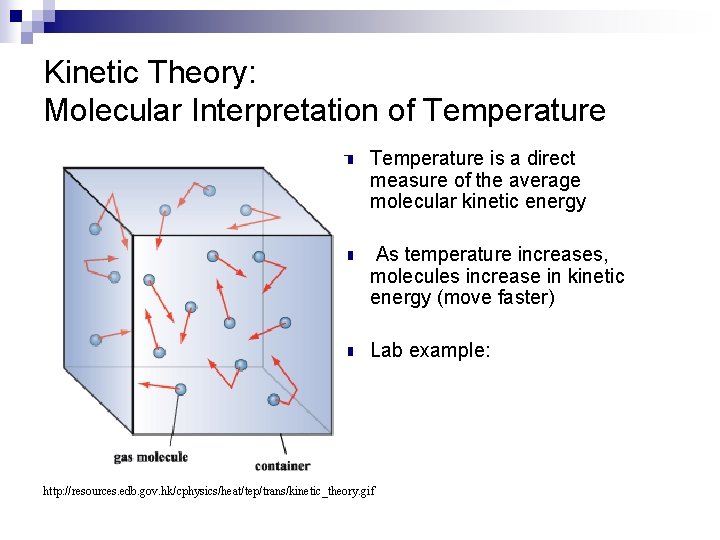
Kinetic Theory: Molecular Interpretation of Temperature n Temperature is a direct measure of the average molecular kinetic energy n As temperature increases, molecules increase in kinetic energy (move faster) n Lab example: http: //resources. edb. gov. hk/cphysics/heat/tep/trans/kinetic_theory. gif

Heat is a form of energy (units= ) n Also called ____ energy n Heat moves from an object/area of high temperature to an object/area of low temperature until thermal equilibrium is reached. n

n Which object has the higher temperature, your hand or the ice? In which direction will heat flow? n Why? n
Methods of Heat Transfer n Conduction: direct contact ¨ Example: n Convection: movement in gases and liquids ¨ Example: n hand on stove Convection currents in ocean Radiation: electromagnetic waves ¨ Example: microwaves in microwaves
Thermal Expansion http: //www. wbacorp. com/img/products/watertite. jpg As temperature increases, molecules within a substance move faster (kinetic theory). n As molecules move faster, they collide more frequently causing the substance to expand n EX: Expansion joints on bridges, bimetallic strips in smoke detectors n www. anaheim. net/. . . /fire/com_svc/sm_det. html
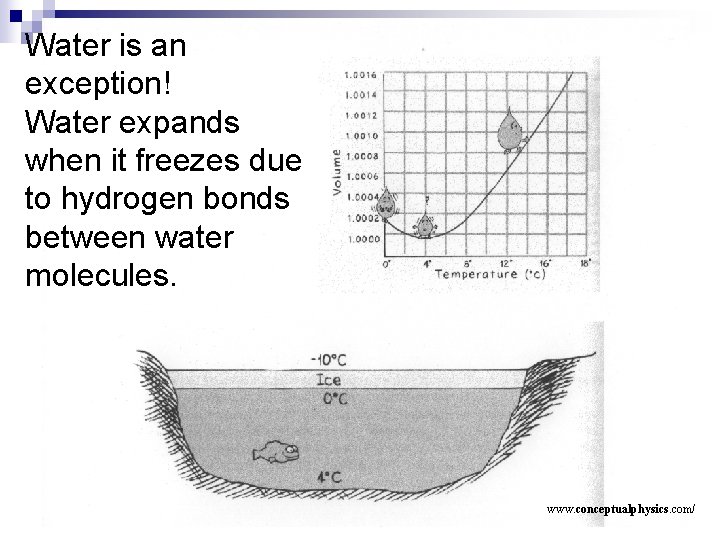
Water is an exception! Water expands when it freezes due to hydrogen bonds between water molecules. www. conceptualphysics. com/

Specific heat n Specific heat is a constant that relates heat and temperature change, per kilogram n Different materials have different specific heat n A low specific heat means heat is conducted through an object quickly

www. fylde. gov. uk/. . . /new-insulation-02. jpg Insulation n Materials with a high specific heat are good insulators n Insulators resist heat flow n Examples: air, paper, fiberglass, styrofoam, wood, glass

Heat Absorption n Station ¨Dark 8 colors absorb more light (energy), and therefore give off more heat (energy) and will have a higher temperature ¨Light colors reflect more light (energy) and have a lower temperature
- Slides: 14