Thermodynamics Temperature Kinetic Theory of Gases Zeroth Law















- Slides: 32

Thermodynamics熱力學 溫度 氣體運動論 Temperature Kinetic Theory of Gases





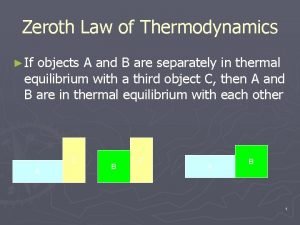
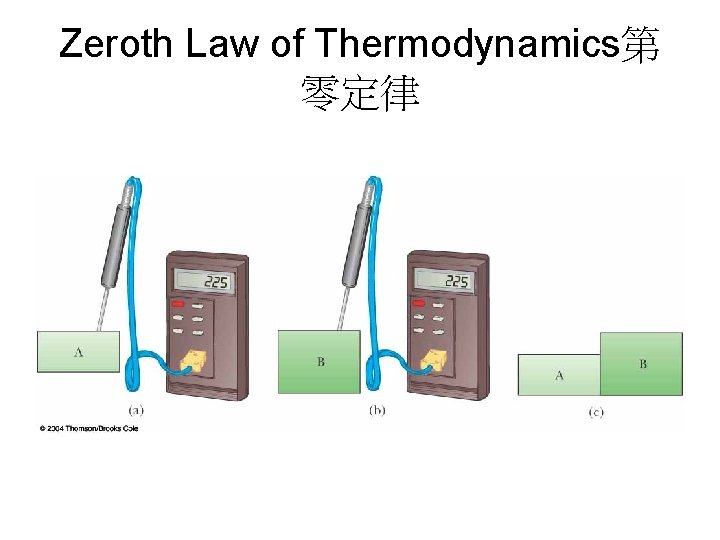
Zeroth Law of Thermodynamics第 零定律

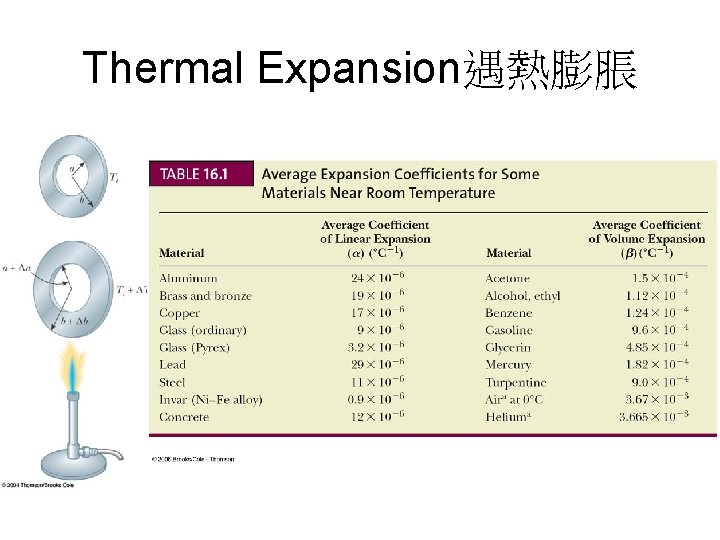
Thermometers溫度計設計的原理 • 物理性質隨溫度而改變 – – – The volume of a liquid The length of a solid The pressure of a gas at a constant volume The volume of a gas at a constant pressure The electric resistance of a conductor The color of an object • A temperature scale can be established on the basis of any of these physical properties

Thermometer, Liquid in Glass

Calibrating a Thermometer溫度計 的校準 • 與衡溫的系統做熱接觸 1. A mixture of ice and water at atmospheric pressure Called the ice point or freezing point of water 2. A mixture of water and steam in equilibrium Called the steam point or boiling point of water

Problems with Liquid-in-Glass Thermometers • An alcohol thermometer and a mercury thermometer may agree only at the calibration points • The discrepancies between thermometers are especially large when the temperatures being measured are far from the calibration points

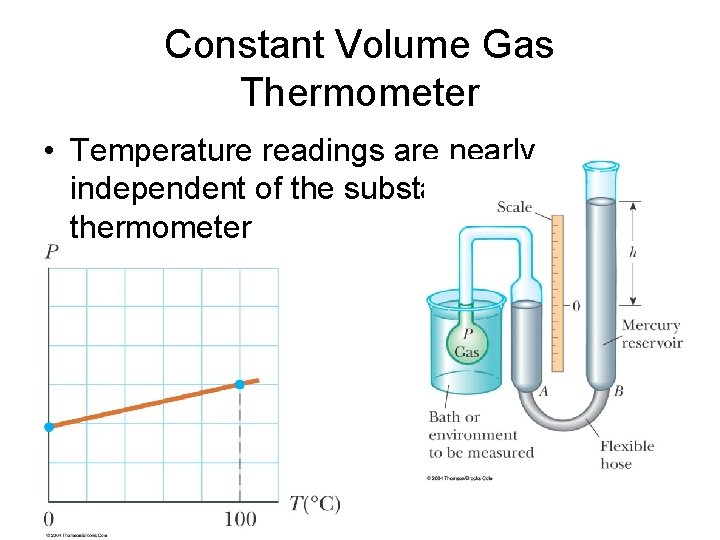
Constant Volume Gas Thermometer • Temperature readings are nearly independent of the substance used in thermometer

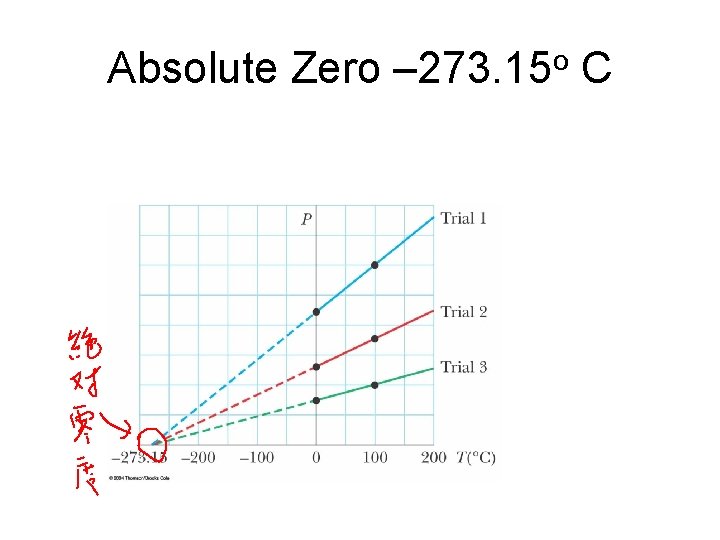
Absolute Zero – 273. 15 o C

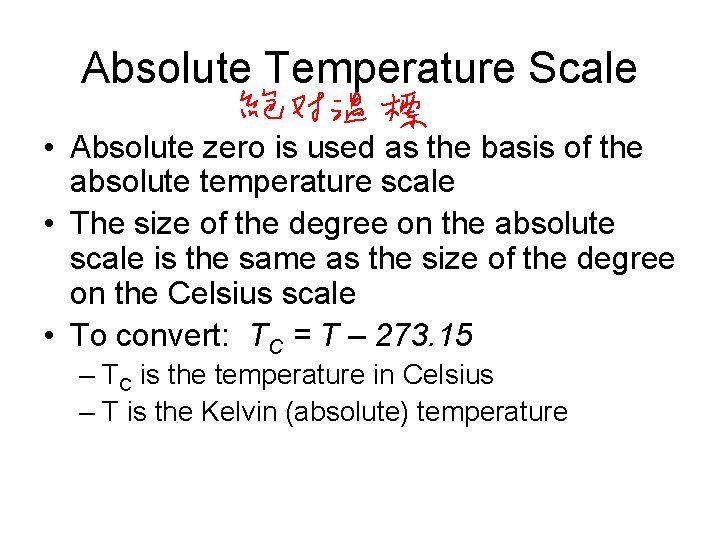
Absolute Temperature Scale • Absolute zero is used as the basis of the absolute temperature scale • The size of the degree on the absolute scale is the same as the size of the degree on the Celsius scale • To convert: TC = T – 273. 15 – TC is the temperature in Celsius – T is the Kelvin (absolute) temperature

Absolute Temperature Scale, 2 • The absolute temperature scale is now based on two new fixed points – Adopted in 1954 by the International Committee on Weights and Measures – One point is absolute zero – The other point is the triple point of water • This is the combination of temperature and pressure where ice, water, and steam can all coexist

Absolute Temperature Scale, 3 • The triple point of water occurs at 0. 01 o C and 4. 58 mm of mercury • This temperature was set to be 273. 16 on the absolute temperature scale – This made the old absolute scale agree closely with the new one – The unit of the absolute scale is the kelvin

Absolute Temperature Scale, 4 • The absolute scale is also called the Kelvin scale – Named for William Thomson, Lord Kelvin • The triple point temperature is 273. 16 K – No degree symbol is used with kelvins • The kelvin is defined as 1/273. 16 of the temperature of the triple point of water

Energy at Absolute Zero • According to classical physics, the kinetic energy of the gas molecules would become zero at absolute zero • The molecular motion would cease – Therefore, the molecules would settle out on the bottom of the container • Quantum theory modifies this and shows some residual energy would remain – This energy is called the zero-point energy

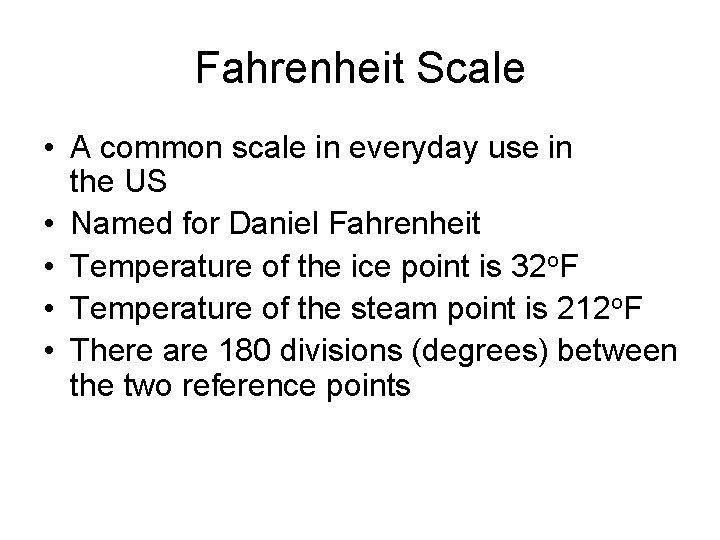
Fahrenheit Scale • A common scale in everyday use in the US • Named for Daniel Fahrenheit • Temperature of the ice point is 32 o. F • Temperature of the steam point is 212 o. F • There are 180 divisions (degrees) between the two reference points

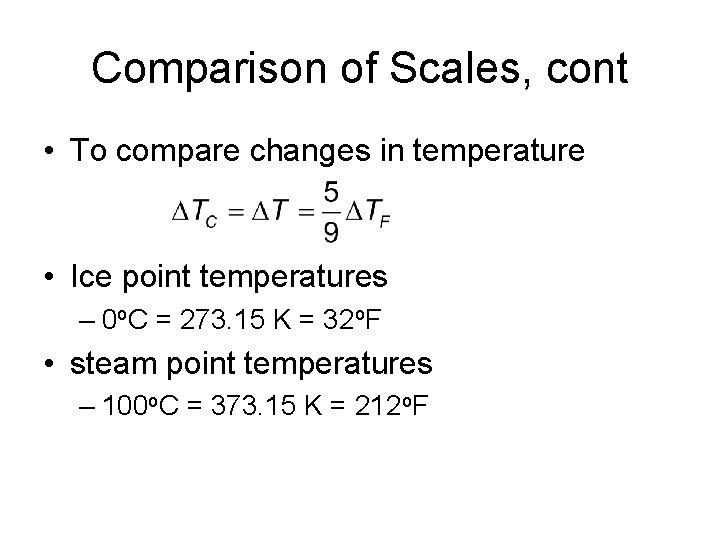
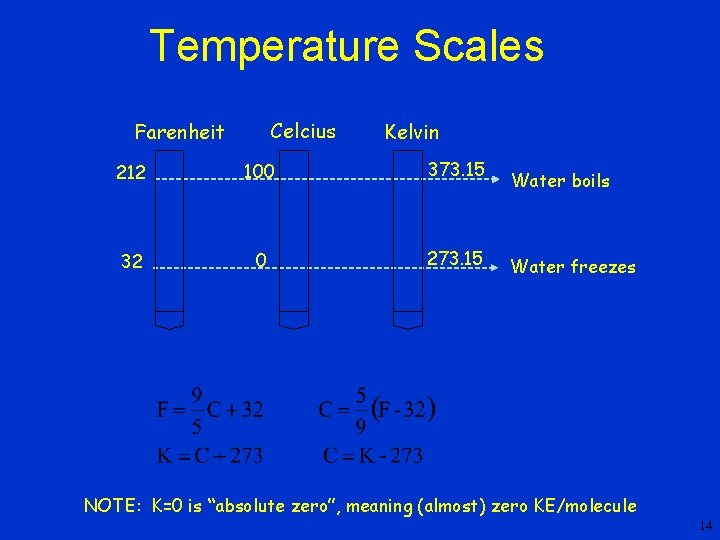
Comparison of Scales, cont • To compare changes in temperature • Ice point temperatures – 0 o. C = 273. 15 K = 32 o. F • steam point temperatures – 100 o. C = 373. 15 K = 212 o. F

Temperature Scales Celcius Farenheit Kelvin 212 100 373. 15 32 0 273. 15 Water boils Water freezes NOTE: K=0 is “absolute zero”, meaning (almost) zero KE/molecule 14


Thermal Expansion遇熱膨脹



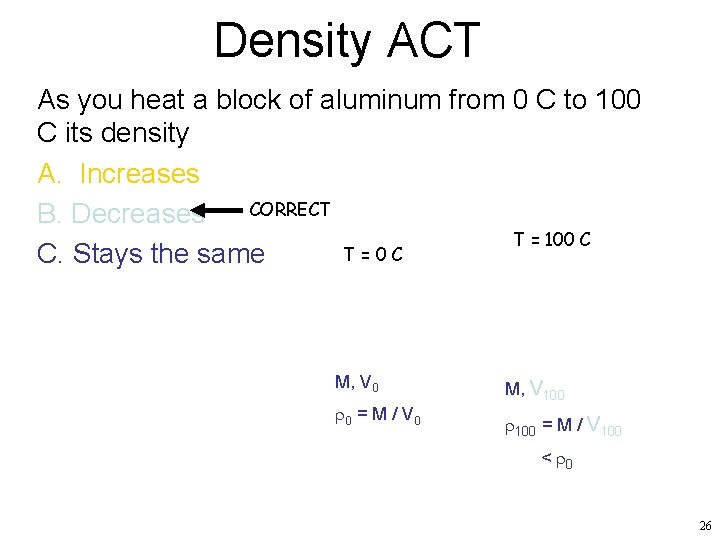
Density ACT As you heat a block of aluminum from 0 C to 100 C its density A. Increases CORRECT B. Decreases T = 100 C T=0 C C. Stays the same M, V 0 r 0 = M / V 0 M, V 100 r 100 = M / V 100 < r 0 26

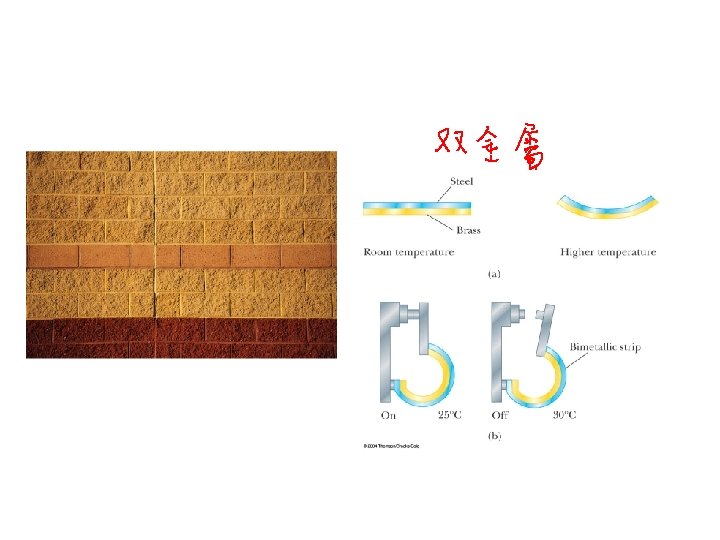
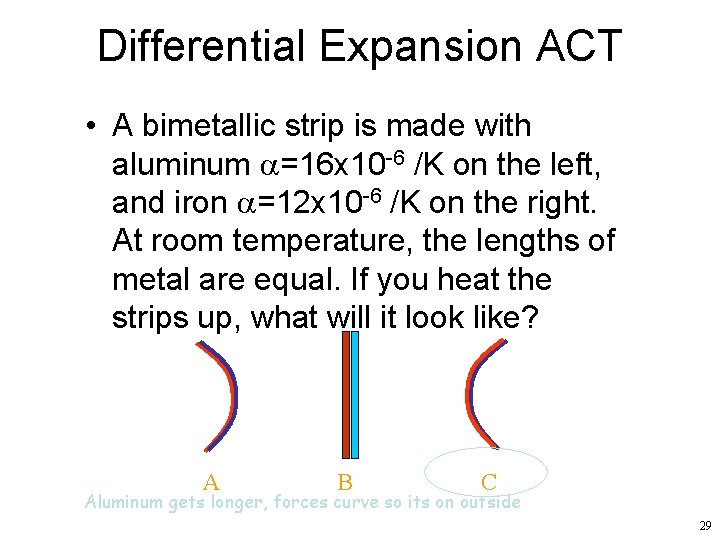
Differential Expansion ACT • A bimetallic strip is made with aluminum a=16 x 10 -6 /K on the left, and iron a=12 x 10 -6 /K on the right. At room temperature, the lengths of metal are equal. If you heat the strips up, what will it look like? A B C Aluminum gets longer, forces curve so its on outside 29

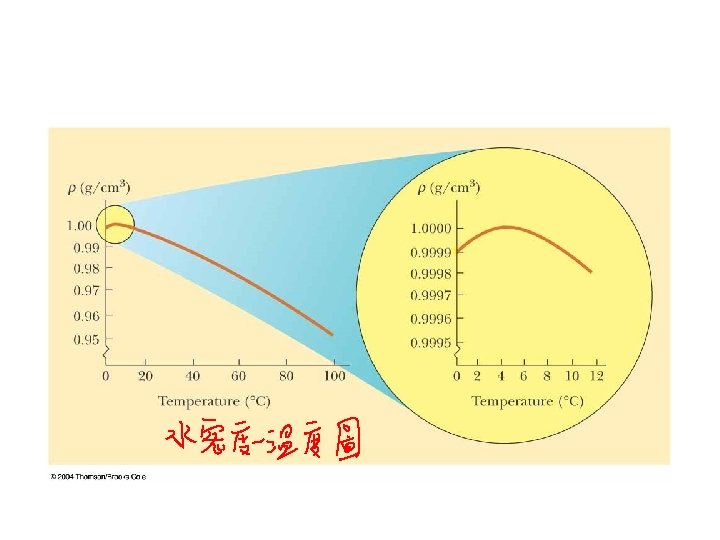
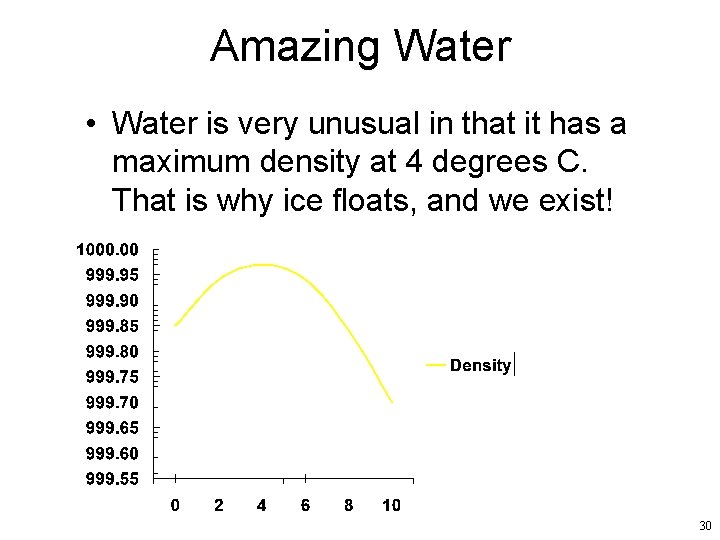
Amazing Water • Water is very unusual in that it has a maximum density at 4 degrees C. That is why ice floats, and we exist! 30

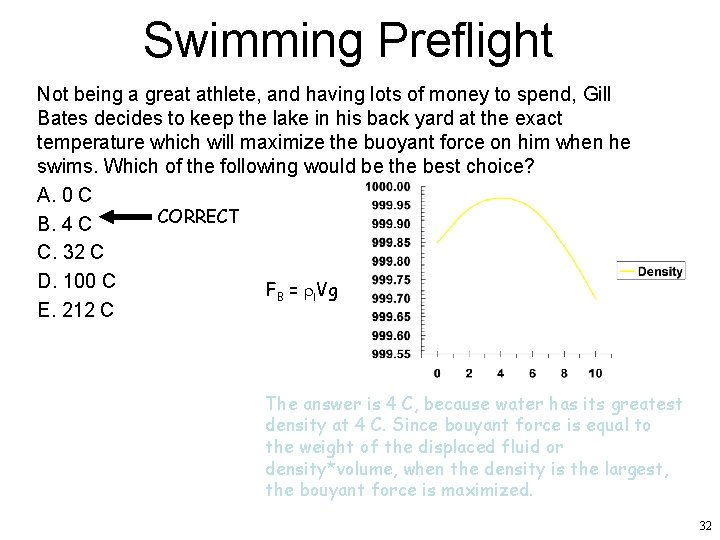
Swimming Preflight Not being a great athlete, and having lots of money to spend, Gill Bates decides to keep the lake in his back yard at the exact temperature which will maximize the buoyant force on him when he swims. Which of the following would be the best choice? A. 0 C CORRECT B. 4 C C. 32 C D. 100 C FB = rl. Vg E. 212 C The answer is 4 C, because water has its greatest density at 4 C. Since bouyant force is equal to the weight of the displaced fluid or density*volume, when the density is the largest, the bouyant force is maximized. 32

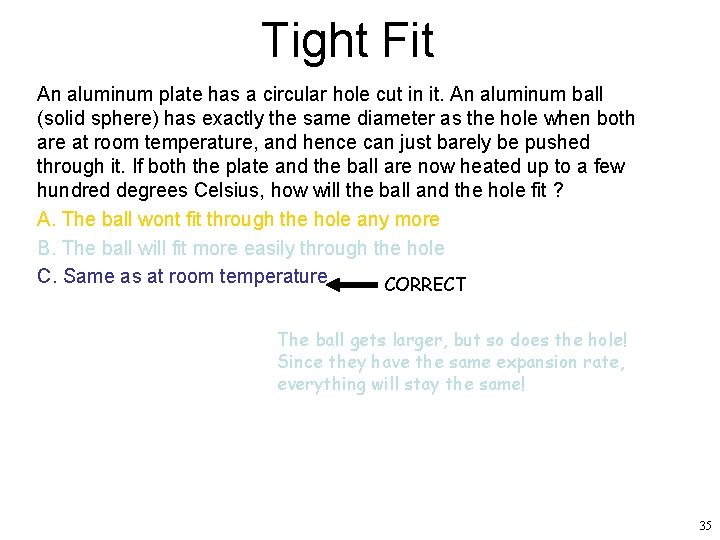
Tight Fit An aluminum plate has a circular hole cut in it. An aluminum ball (solid sphere) has exactly the same diameter as the hole when both are at room temperature, and hence can just barely be pushed through it. If both the plate and the ball are now heated up to a few hundred degrees Celsius, how will the ball and the hole fit ? A. The ball wont fit through the hole any more B. The ball will fit more easily through the hole C. Same as at room temperature CORRECT The ball gets larger, but so does the hole! Since they have the same expansion rate, everything will stay the same! 35

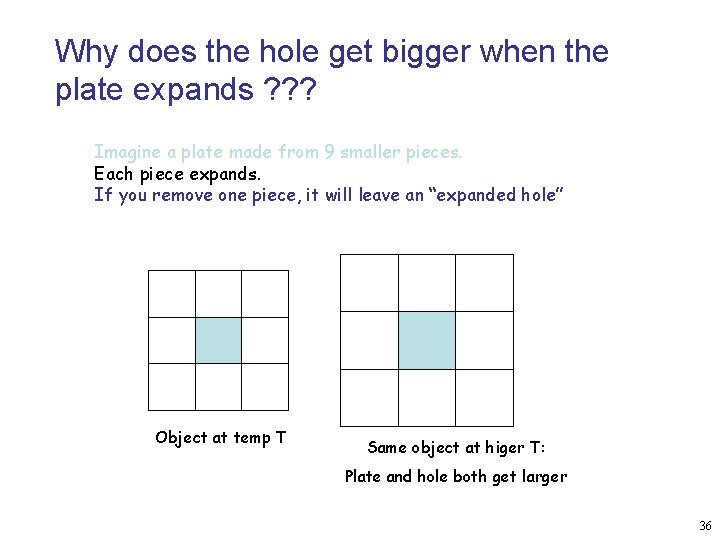
Why does the hole get bigger when the plate expands ? ? ? Imagine a plate made from 9 smaller pieces. Each piece expands. If you remove one piece, it will leave an “expanded hole” Object at temp T Same object at higer T: Plate and hole both get larger 36

Jar Act A cylindrical glass container (b = 28 x 10 -6 k-1) is filled to the brim with water (b = 208 x 10 -6 k-1). If the cup and water are heated 50 C what will happen A) Some water overflows Water expands more than B) Same container, so it overflows. C) Water below rim See example 13. 3 in book 41

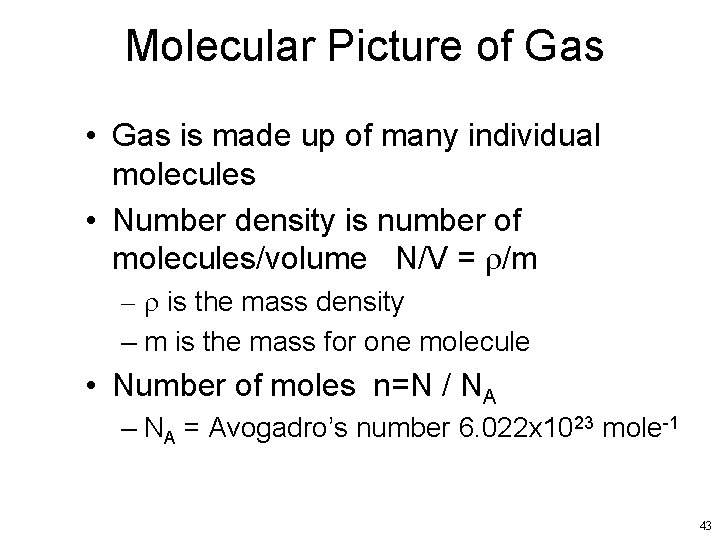
Molecular Picture of Gas • Gas is made up of many individual molecules • Number density is number of molecules/volume N/V = r/m – r is the mass density – m is the mass for one molecule • Number of moles n=N / NA – NA = Avogadro’s number 6. 022 x 1023 mole-1 43
 Thermodynamic
Thermodynamic Zeroth law of thermodynamics statement
Zeroth law of thermodynamics statement State second law of thermodynamics
State second law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics Kinetic molecular model of gases
Kinetic molecular model of gases Kinetic particle theory questions
Kinetic particle theory questions Kinetic theory of gases
Kinetic theory of gases Postulates of kinetic theory
Postulates of kinetic theory Kinetic theory
Kinetic theory Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Postulates of kinetic theory of gases
Postulates of kinetic theory of gases General gas equation is
General gas equation is First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas First order kinetics
First order kinetics Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Temperature is a measure of the average
Temperature is a measure of the average Temperature energy
Temperature energy Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Ferrimagnetism
Ferrimagnetism First law of thermodynamics for open system
First law of thermodynamics for open system Newton's third law of thermodynamics
Newton's third law of thermodynamics Isobaric process formula
Isobaric process formula Brayton cycle
Brayton cycle State second law of thermodynamics
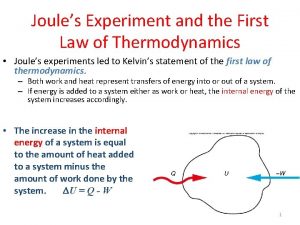
State second law of thermodynamics During joules experiment on first law of thermodynamics *
During joules experiment on first law of thermodynamics * Internal energy in thermodynamics definition
Internal energy in thermodynamics definition In a flow process the work transfer may be of which type
In a flow process the work transfer may be of which type P v t relationship in adiabatic process
P v t relationship in adiabatic process Law of thermodynamics
Law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics