Thermodynamics of defect formation Both equations agree with
Thermodynamics of defect formation
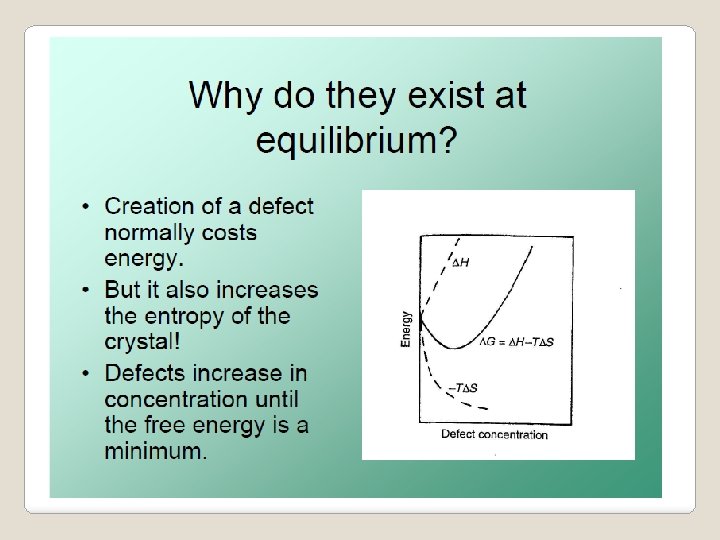

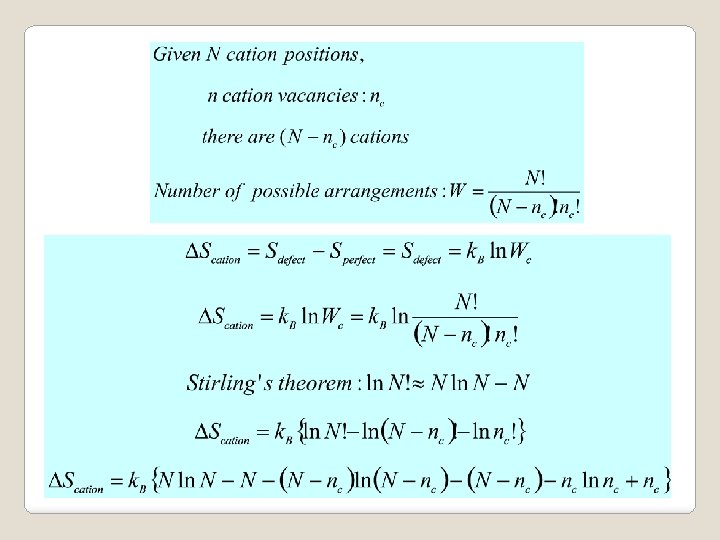
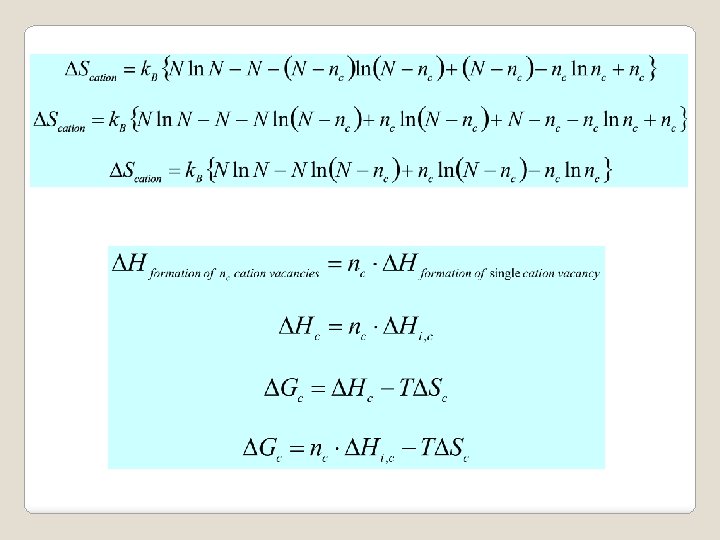


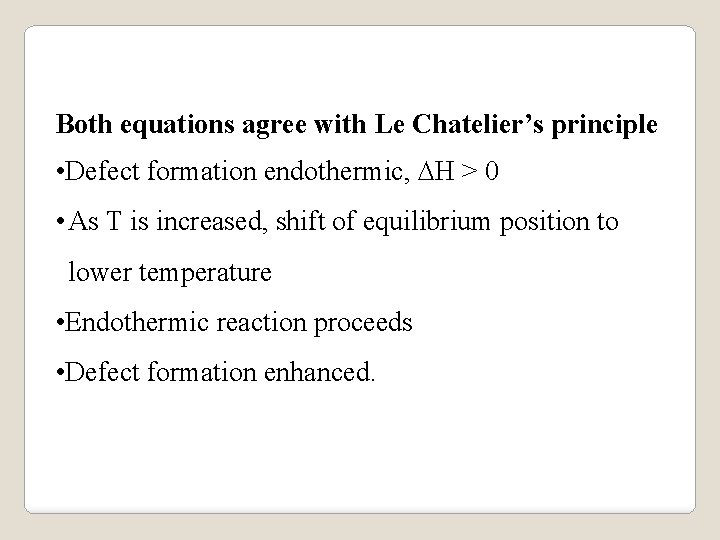
Both equations agree with Le Chatelier’s principle • Defect formation endothermic, DH > 0 • As T is increased, shift of equilibrium position to lower temperature • Endothermic reaction proceeds • Defect formation enhanced.

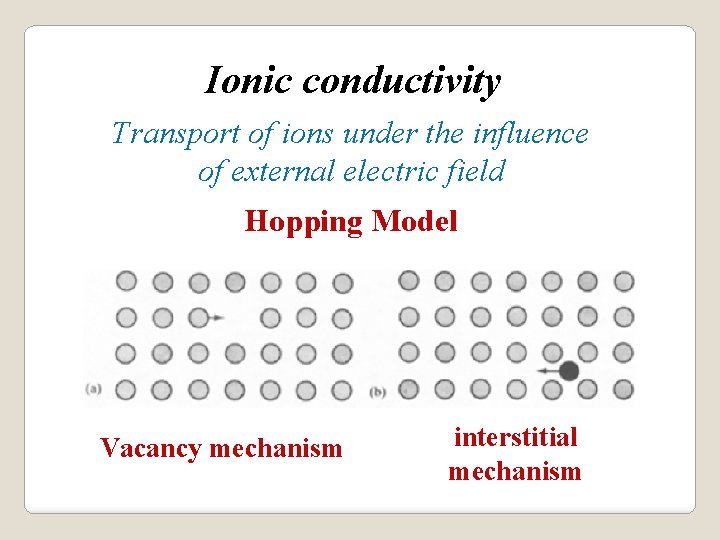
Ionic conductivity Transport of ions under the influence of external electric field Hopping Model Vacancy mechanism interstitial mechanism
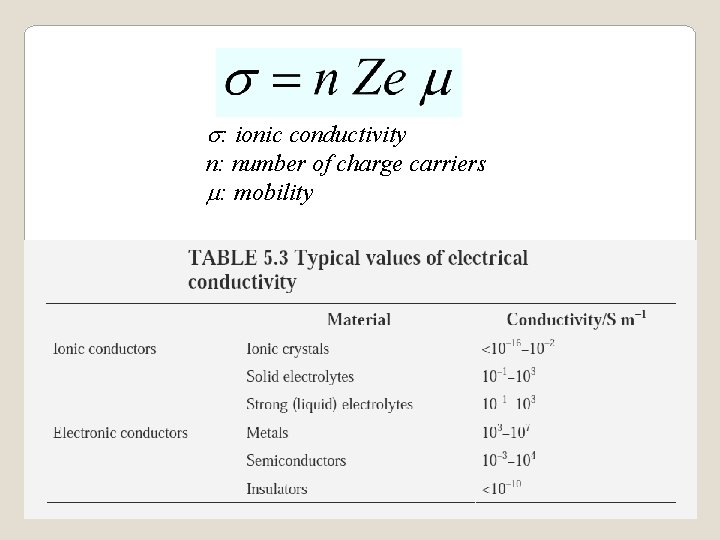
s: ionic conductivity n: number of charge carriers m: mobility
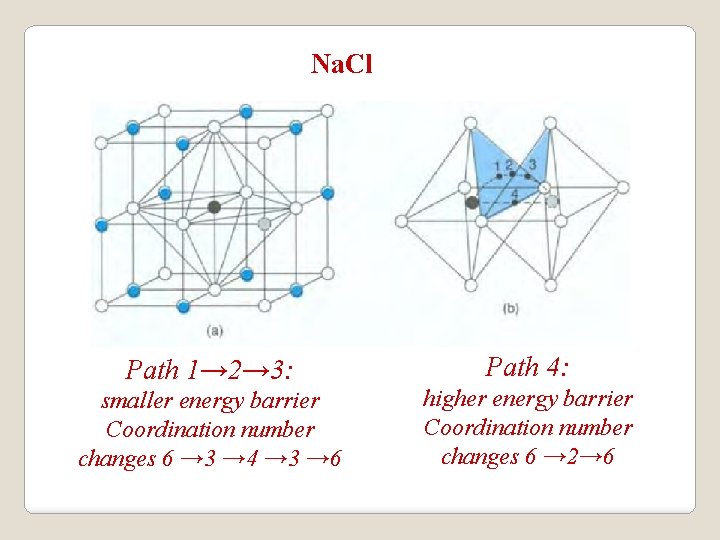
Na. Cl Path 1→ 2→ 3: smaller energy barrier Coordination number changes 6 → 3 → 4 → 3 → 6 Path 4: higher energy barrier Coordination number changes 6 → 2→ 6
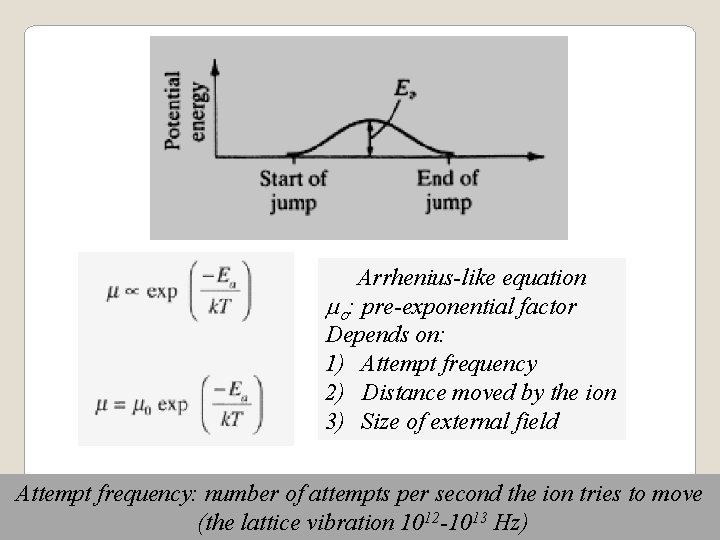
Arrhenius-like equation mo: pre-exponential factor Depends on: 1) Attempt frequency 2) Distance moved by the ion 3) Size of external field Attempt frequency: number of attempts per second the ion tries to move (the lattice vibration 1012 -1013 Hz)
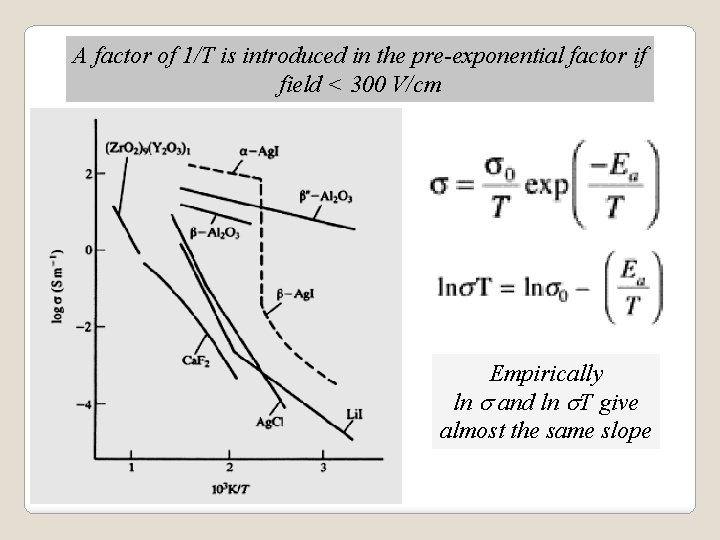
A factor of 1/T is introduced in the pre-exponential factor if field < 300 V/cm Empirically ln s and ln s. T give almost the same slope
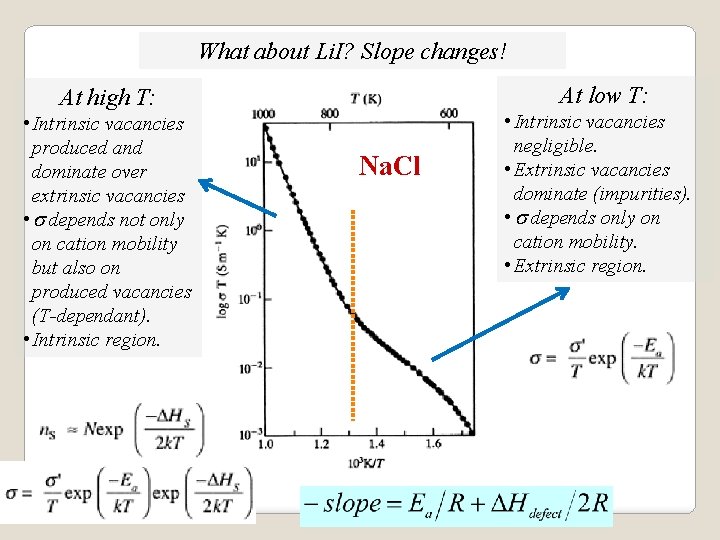
What about Li. I? Slope changes! At low T: At high T: • Intrinsic vacancies produced and dominate over extrinsic vacancies • s depends not only on cation mobility but also on produced vacancies (T-dependant). • Intrinsic region. Na. Cl • Intrinsic vacancies negligible. • Extrinsic vacancies dominate (impurities). • s depends only on cation mobility. • Extrinsic region.
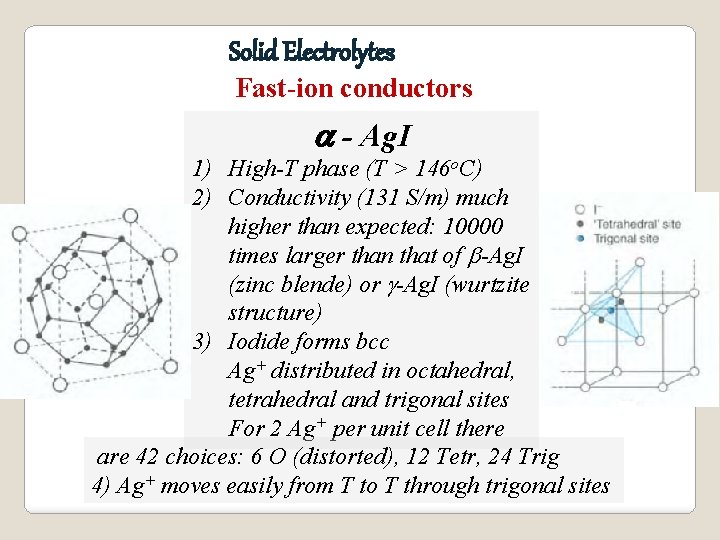
Solid Electrolytes Fast-ion conductors a - Ag. I 1) High-T phase (T > 146 o. C) 2) Conductivity (131 S/m) much higher than expected: 10000 times larger than that of b-Ag. I (zinc blende) or g-Ag. I (wurtzite structure) 3) Iodide forms bcc Ag+ distributed in octahedral, tetrahedral and trigonal sites For 2 Ag+ per unit cell there are 42 choices: 6 O (distorted), 12 Tetr, 24 Trig 4) Ag+ moves easily from T to T through trigonal sites
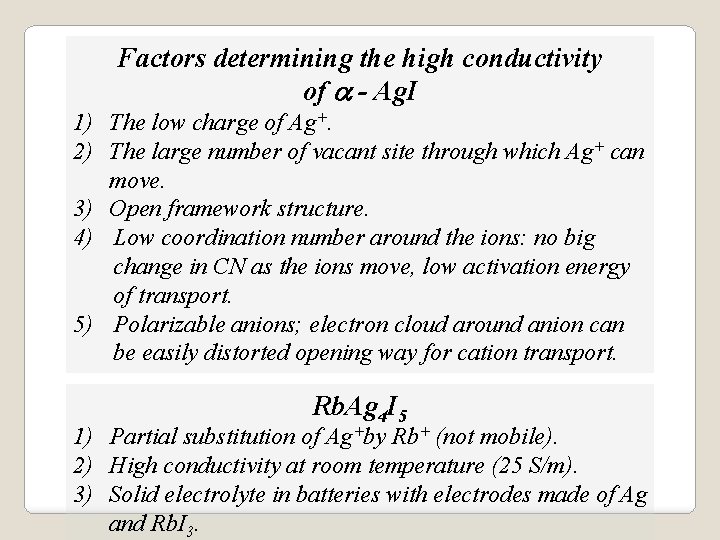
Factors determining the high conductivity of a - Ag. I 1) The low charge of Ag+. 2) The large number of vacant site through which Ag+ can move. 3) Open framework structure. 4) Low coordination number around the ions: no big change in CN as the ions move, low activation energy of transport. 5) Polarizable anions; electron cloud around anion can be easily distorted opening way for cation transport. Rb. Ag 4 I 5 1) Partial substitution of Ag+by Rb+ (not mobile). 2) High conductivity at room temperature (25 S/m). 3) Solid electrolyte in batteries with electrodes made of Ag and Rb. I 3.

oxygen-ion conductors Stabilized Zirconia (Zr. O 2) 1) Fluorite structure of Zr. O 2 stable only at very high temperatures (>2370 o. C). 2) Stabilized at room temperature by the addition of yttrium oxide or calcium oxide. 3) Ca 2+ replaces Zr 4+ without changing the structure. 4) To conserve electroneutrality, oxygen vacancies generated , fast ion conductor. 5) Yttrium stabilized zirconia (YSZ) is the usual material used in solid oxide fuel cell (SOFCs). 6) Two of the best oxygen ion conductors are scandia (Sc 2 O 3)stabilized zirconia and gadolinia (Gd 2 O 3) stabilized ceria (Ce. O 2) because of the similar cation size (minimal distortion of structure).
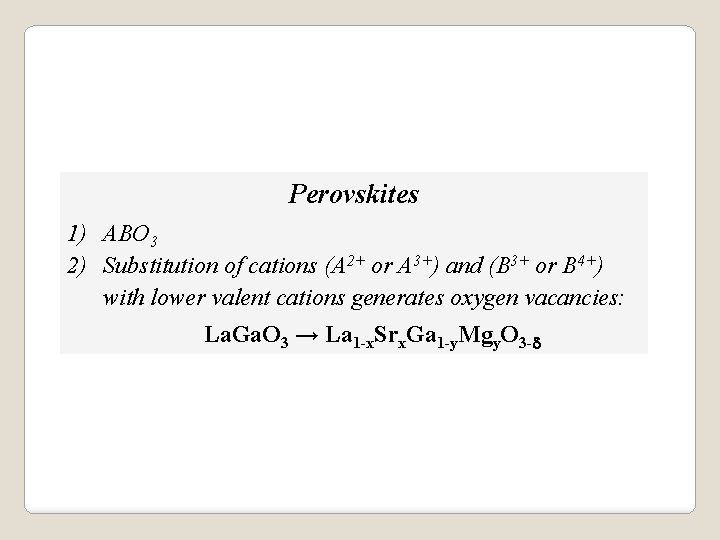
Perovskites 1) ABO 3 2) Substitution of cations (A 2+ or A 3+) and (B 3+ or B 4+) with lower valent cations generates oxygen vacancies: La. Ga. O 3 → La 1 -x. Srx. Ga 1 -y. Mgy. O 3 -d
Batteries Solid state batteries Advantages 1) Solid electrolyte (spill-safe). 2) Perform over a wide range of temperature. 3) Long shelf-time. 4) Small size, light weight. 5) Mobiles, laptops, backup power supplies (UPS), hybrid cars.
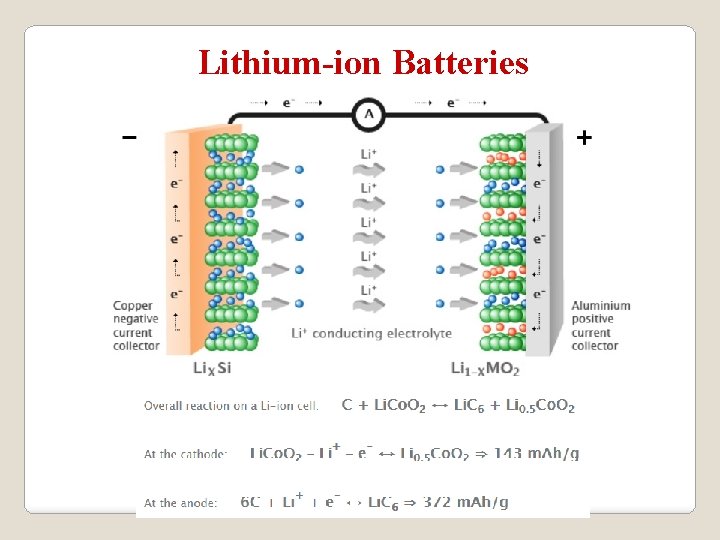
Lithium-ion Batteries

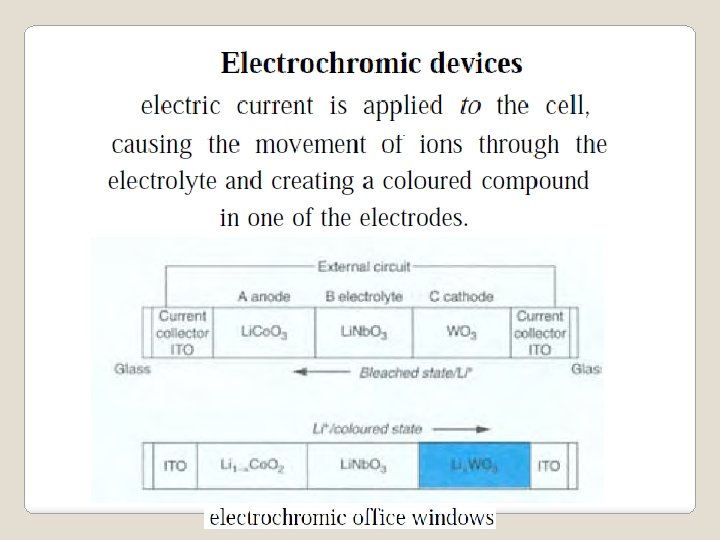
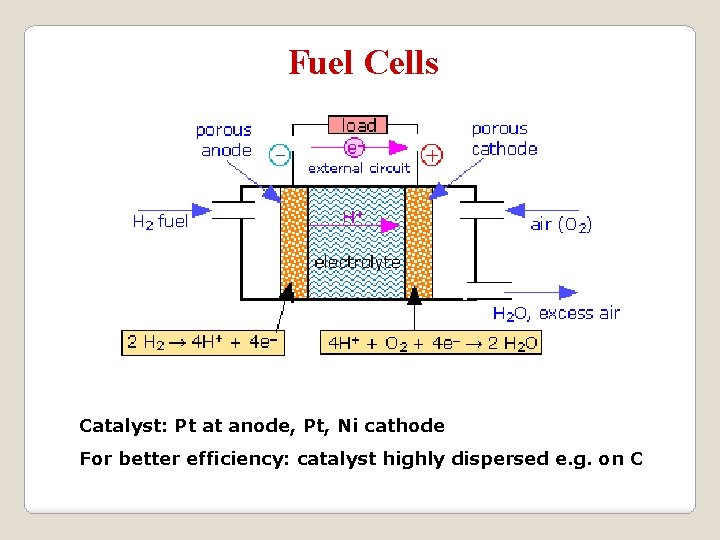
Fuel Cells Catalyst: Pt at anode, Pt, Ni cathode For better efficiency: catalyst highly dispersed e. g. on C
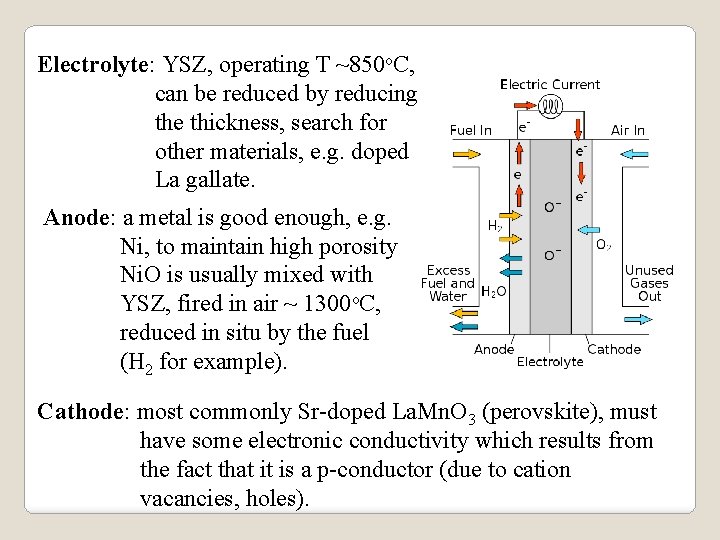
Electrolyte: YSZ, operating T ~850 o. C, can be reduced by reducing the thickness, search for other materials, e. g. doped La gallate. Anode: a metal is good enough, e. g. Ni, to maintain high porosity Ni. O is usually mixed with YSZ, fired in air ~ 1300 o. C, reduced in situ by the fuel (H 2 for example). Cathode: most commonly Sr-doped La. Mn. O 3 (perovskite), must have some electronic conductivity which results from the fact that it is a p-conductor (due to cation vacancies, holes).
- Slides: 30