Thermodynamics l a system Some portion of the


- Slides: 18

Thermodynamics l a system: Some portion of the universe that you wish to study l The surroundings: The adjacent part of the universe outside the system Changes in a system are associated with the transfer of energy Natural systems tend toward states of minimum energy

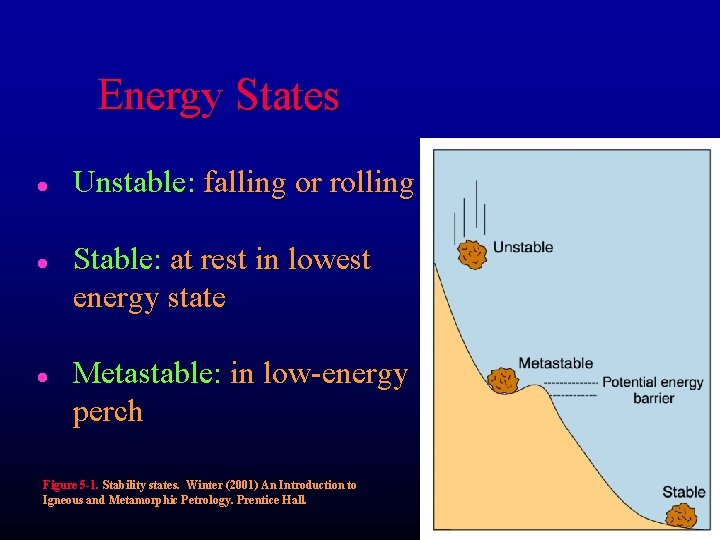
Energy States l l l Unstable: falling or rolling Stable: at rest in lowest energy state Metastable: in low-energy perch Figure 5 -1. Stability states. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.

Gibbs Free Energy Gibbs free energy is a measure of chemical energy All chemical systems tend naturally toward states of minimum Gibbs free energy G = H - TS Where: G = Gibbs Free Energy H = Enthalpy (heat content) T = Temperature in Kelvins S = Entropy (can think of as randomness)

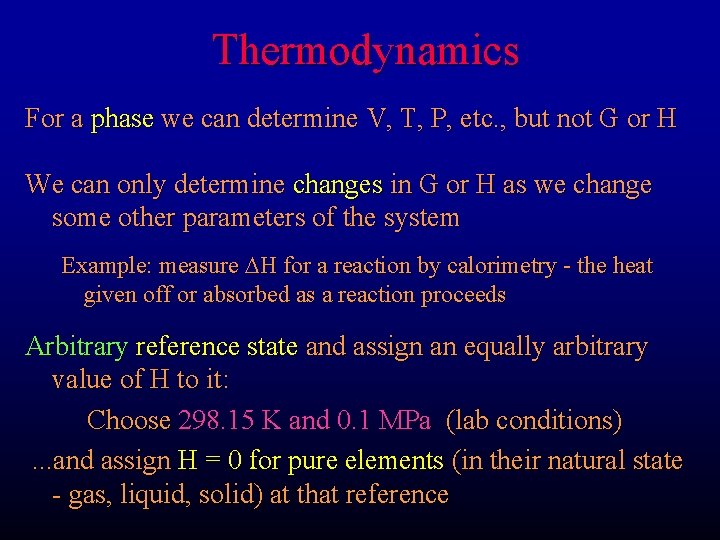
Thermodynamics a Phase: a mechanically separable portion of a system F Mineral F Liquid F Vapor a Reaction: some change in the nature or types of phases in a system reactions are written in the form: reactants = products

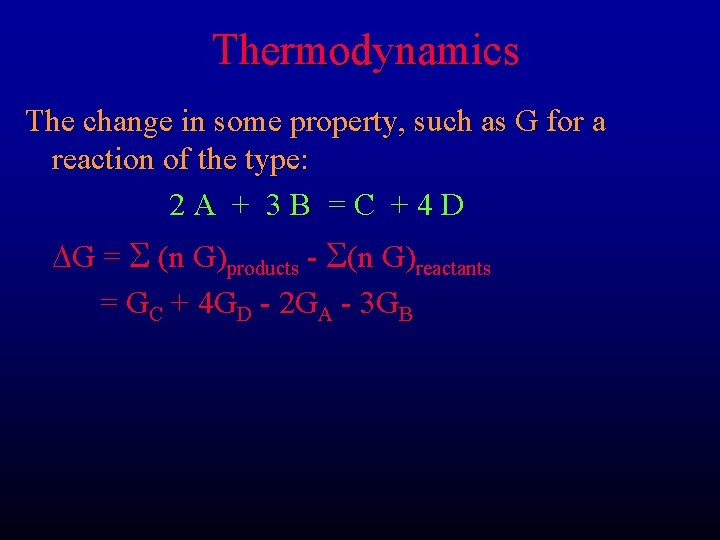
Thermodynamics The change in some property, such as G for a reaction of the type: 2 A + 3 B =C +4 D DG = S (n G)products - S(n G)reactants = GC + 4 GD - 2 GA - 3 GB

Thermodynamics For a phase we can determine V, T, P, etc. , but not G or H We can only determine changes in G or H as we change some other parameters of the system Example: measure DH for a reaction by calorimetry - the heat given off or absorbed as a reaction proceeds Arbitrary reference state and assign an equally arbitrary value of H to it: Choose 298. 15 K and 0. 1 MPa (lab conditions). . . and assign H = 0 for pure elements (in their natural state - gas, liquid, solid) at that reference

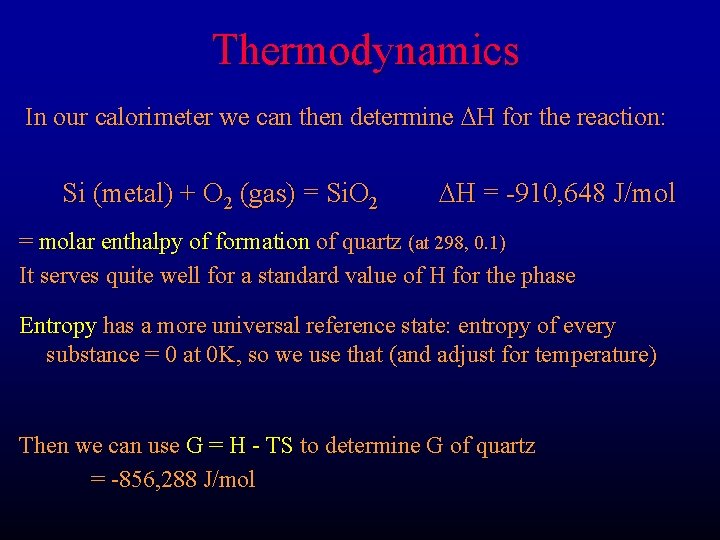
Thermodynamics In our calorimeter we can then determine DH for the reaction: Si (metal) + O 2 (gas) = Si. O 2 DH = -910, 648 J/mol = molar enthalpy of formation of quartz (at 298, 0. 1) It serves quite well for a standard value of H for the phase Entropy has a more universal reference state: entropy of every substance = 0 at 0 K, so we use that (and adjust for temperature) Then we can use G = H - TS to determine G of quartz = -856, 288 J/mol

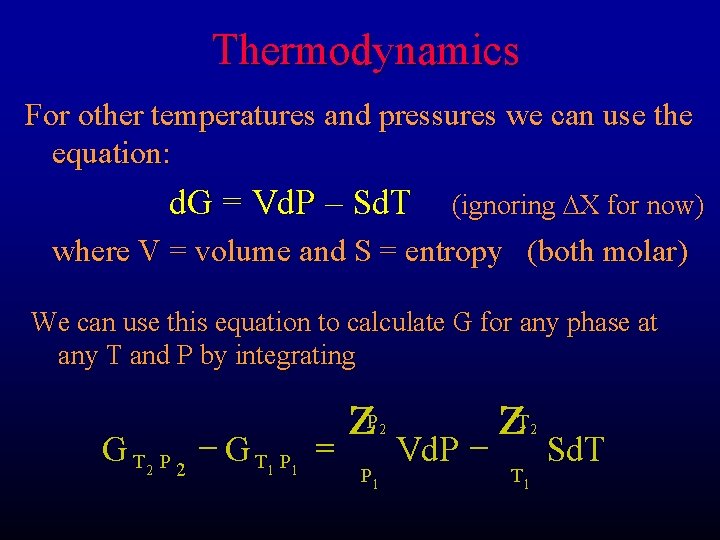
Thermodynamics For other temperatures and pressures we can use the equation: d. G = Vd. P – Sd. T (ignoring DX for now) where V = volume and S = entropy (both molar) We can use this equation to calculate G for any phase at any T and P by integrating GT 2 P 2 - GT 1 P 1 = z P 2 P 1 Vd. P - z T 2 T 1 Sd. T

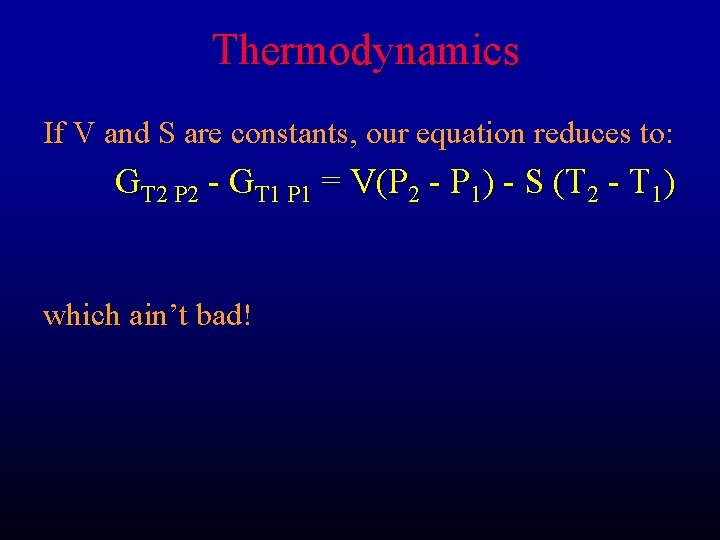
Thermodynamics If V and S are constants, our equation reduces to: GT 2 P 2 - GT 1 P 1 = V(P 2 - P 1) - S (T 2 - T 1) which ain’t bad!

Thermodynamics In Worked Example 1 we used GT 2 P 2 - GT 1 P 1 = V(P 2 - P 1) - S (T 2 - T 1) and G 298, 0. 1 = -856, 288 J/mol to calculate G for quartz at several temperatures and pressures Low quartz Eq. 1 SUPCRT P (MPa) T (C) G (J) eq. 1 G(J) V (cm 3) S (J/K) 0. 1 25 -856, 288 -856, 648 22. 69 41. 36 500 25 -844, 946 -845, 362 22. 44 40. 73 0. 1 500 -875, 982 -890, 601 23. 26 96. 99 500 -864, 640 -879, 014 23. 07 96. 36 Agreement is quite good (< 2% for change of 500 o and 500 MPa or 17 km)

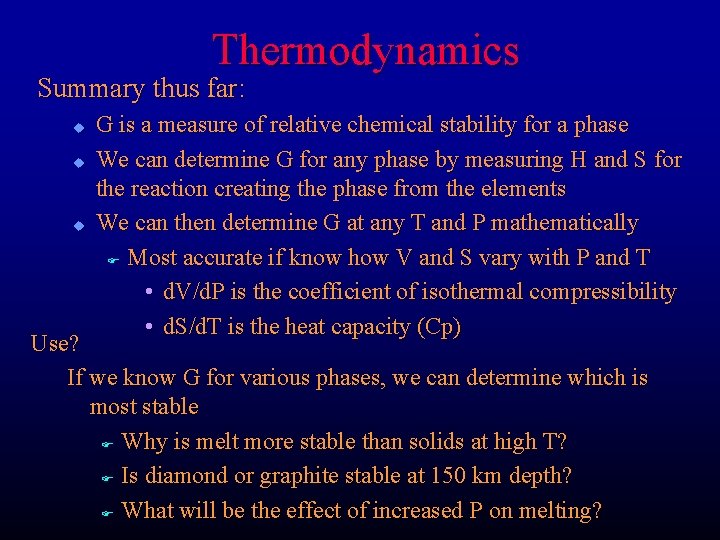
Thermodynamics Summary thus far: u u u G is a measure of relative chemical stability for a phase We can determine G for any phase by measuring H and S for the reaction creating the phase from the elements We can then determine G at any T and P mathematically F Most accurate if know how V and S vary with P and T • d. V/d. P is the coefficient of isothermal compressibility • d. S/d. T is the heat capacity (Cp) Use? If we know G for various phases, we can determine which is most stable F Why is melt more stable than solids at high T? F Is diamond or graphite stable at 150 km depth? F What will be the effect of increased P on melting?

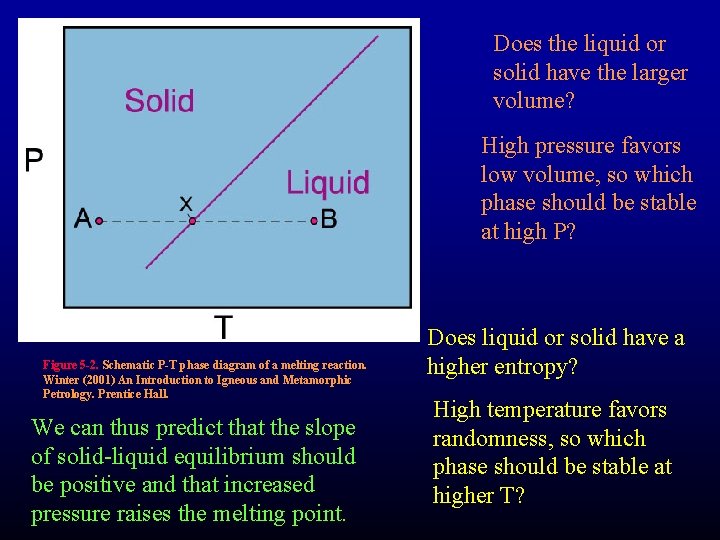
Does the liquid or solid have the larger volume? High pressure favors low volume, so which phase should be stable at high P? Figure 5 -2. Schematic P-T phase diagram of a melting reaction. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall. We can thus predict that the slope of solid-liquid equilibrium should be positive and that increased pressure raises the melting point. Does liquid or solid have a higher entropy? High temperature favors randomness, so which phase should be stable at higher T?

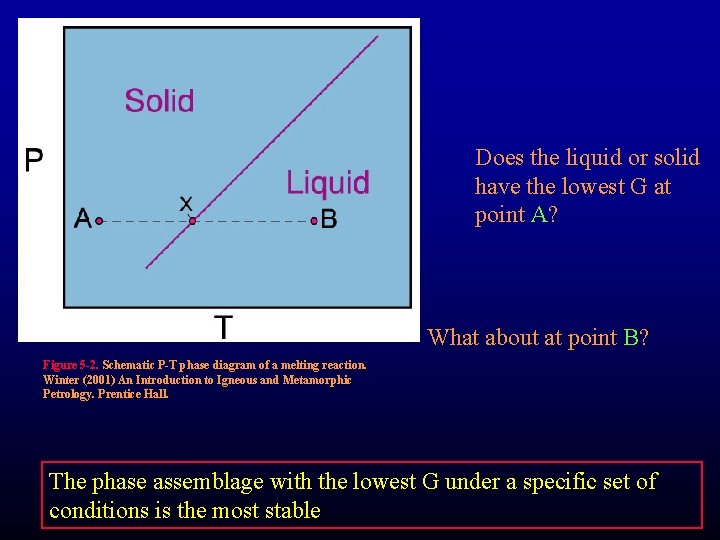
Does the liquid or solid have the lowest G at point A? What about at point B? Figure 5 -2. Schematic P-T phase diagram of a melting reaction. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall. The phase assemblage with the lowest G under a specific set of conditions is the most stable

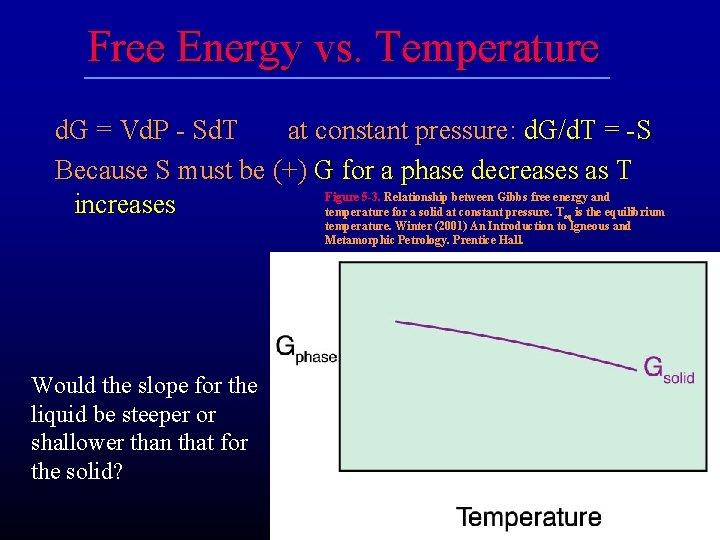
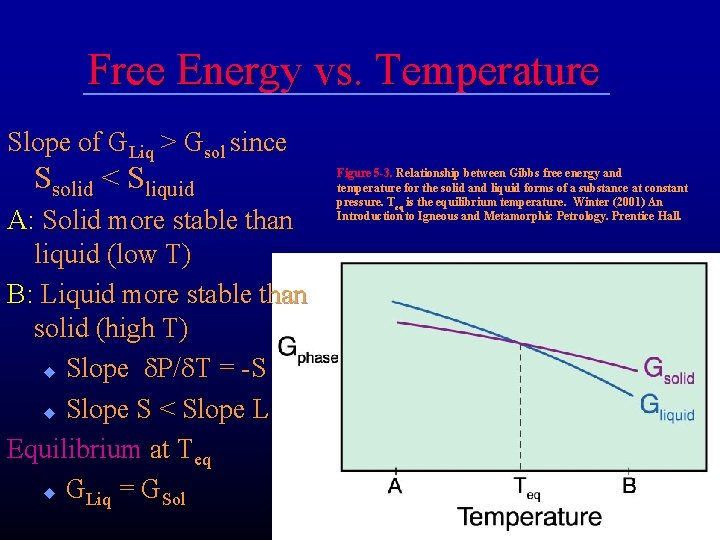
Free Energy vs. Temperature d. G = Vd. P - Sd. T at constant pressure: d. G/d. T = -S Because S must be (+) G for a phase decreases as T Figure 5 -3. Relationship between Gibbs free energy and increases temperature for a solid at constant pressure. T is the equilibrium eq temperature. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall. Would the slope for the liquid be steeper or shallower than that for the solid?

Free Energy vs. Temperature Slope of GLiq > Gsol since Ssolid < Sliquid A: Solid more stable than liquid (low T) B: Liquid more stable than solid (high T) u Slope d. P/d. T = -S u Slope S < Slope L Equilibrium at Teq u GLiq = GSol Figure 5 -3. Relationship between Gibbs free energy and temperature for the solid and liquid forms of a substance at constant pressure. Teq is the equilibrium temperature. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.

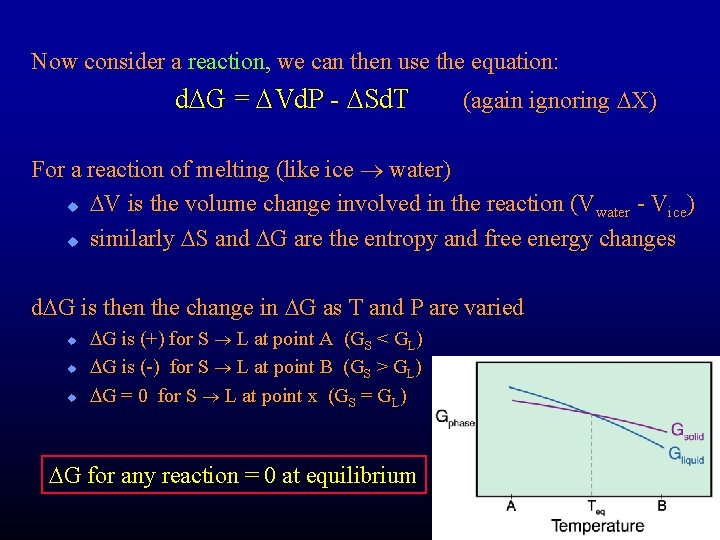
Now consider a reaction, we can then use the equation: d. DG = DVd. P - DSd. T (again ignoring DX) For a reaction of melting (like ice water) u DV is the volume change involved in the reaction ( Vwater - Vice) u similarly DS and DG are the entropy and free energy changes d. DG is then the change in DG as T and P are varied u u u DG is (+) for S L at point A (GS < GL) DG is (-) for S L at point B (GS > GL) DG = 0 for S L at point x (GS = GL) DG for any reaction = 0 at equilibrium

Figures I don’t use in class Figure 5 -4. Relationship between Gibbs free energy and pressure for the solid and liquid forms of a substance at constant temperature. Peq is the equilibrium pressure. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.

Figures I don’t use in class Figure 5 -5. Piston-and-cylinder apparatus to compress a gas. Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.
 They say sometimes you win some
They say sometimes you win some Sometimes you win some sometimes you lose some
Sometimes you win some sometimes you lose some Ice cream uncountable
Ice cream uncountable Contact and noncontact forces
Contact and noncontact forces Some say the world will end in fire some say in ice
Some say the world will end in fire some say in ice Some say the world will end in fire some say in ice
Some say the world will end in fire some say in ice Some trust in chariots and some in horses song
Some trust in chariots and some in horses song Steady flow energy equation thermodynamics
Steady flow energy equation thermodynamics Homogeneous system in thermodynamics
Homogeneous system in thermodynamics Difference between an open and closed system
Difference between an open and closed system Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất