Thermodynamics Heat disorder spontaneity Energy The capacity to













































- Slides: 63

Thermodynamics Heat, disorder, spontaneity

Energy • The capacity to perform work –often measured as heat

Energy • A tub is filled with water at 35°C –Dip a cup into the water and fill it. –What is the temperature of the water in the cup?

Energy • Which amount of water, that in the tub or in the cup, can melt the greater amount of ice during the same time frame?

Energy • Two substances may have the same temperature but different amounts of heat energy.

Energy • Temperature is the measure of average KE of a substance

Energy • Heat is the measure of the total energy transferred from an object with a higher temperature to an object with a lower temperature.

Energy • Heat is measured in either Joules (J) or calories (cal) • A calorie is defined as the amount of heat needed to raise 1 g of water 1°C. • 1 cal = 4. 18 J

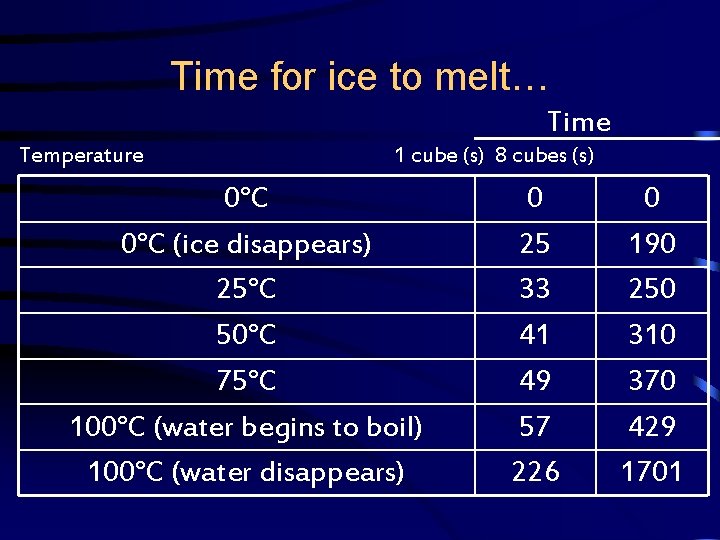
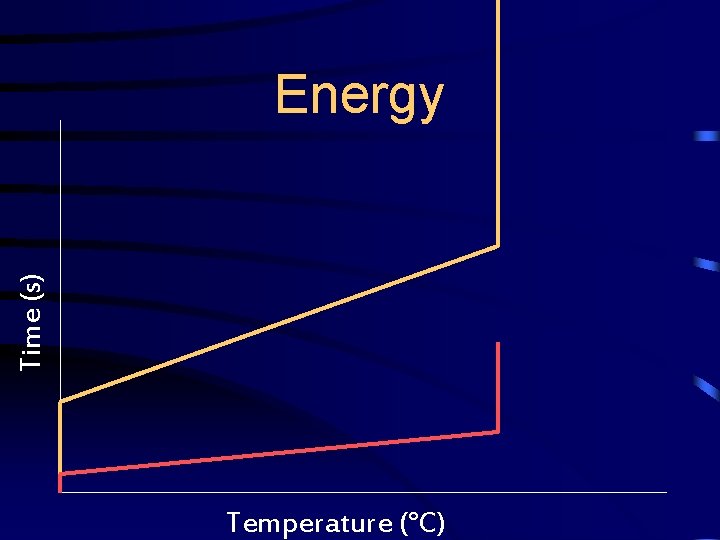
Energy • Graph the following data for two experiments on the same hand-drawn graph.

Time for ice to melt… Time Temperature 1 cube (s) 8 cubes (s) 0°C (ice disappears) 25°C 50°C 75°C 100°C (water begins to boil) 100°C (water disappears) 0 25 33 41 49 57 226 0 190 250 310 370 429 1701

Time (s) Energy Temperature (°C)

Specific Heat Capacity • the amount of heat energy required to raise the temperature of one gram of a substance by one degree Celsius • Measured in J/g°C or cal/g°C

Specific Heat Capacity • When a substance’s SHC (or C) is greater, more heat is required to make that substance equal in temperature to a substance with a lesser SHC

Specific Heat Capacity • Which has the greater SHC, silicone or iron? heat = ( T)(mass)(SHC)

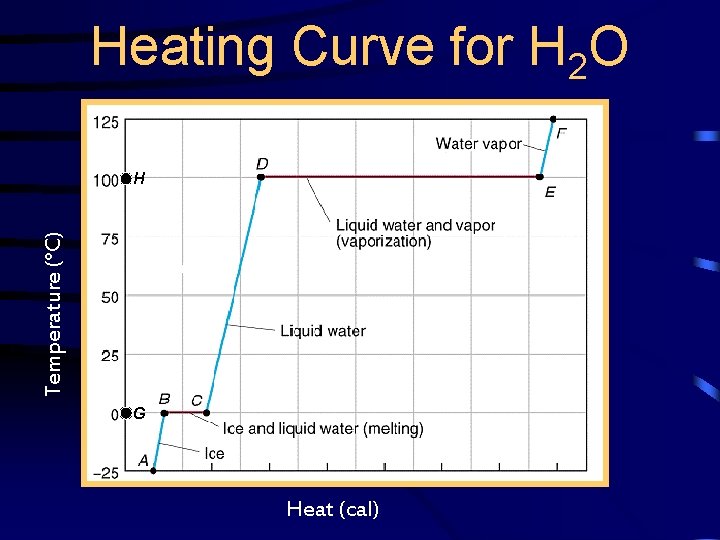
Heating Curve for H 2 O Temperature (°C) H G G Heat (cal)

Heating Curve for H 2 O • BC has value of 80 cal/g –Known as the heat of fusion (s l) or heat of solidification (l s) • DE has a value of 540 cal/g –Known as the heat of vaporization (l g) or heat of condensation (g l)

Heating Curve for H 2 O • G has a value of 0°C –known as the melting point or the freezing point • H has a value of 100°C –Known as the boiling point or the condensation point

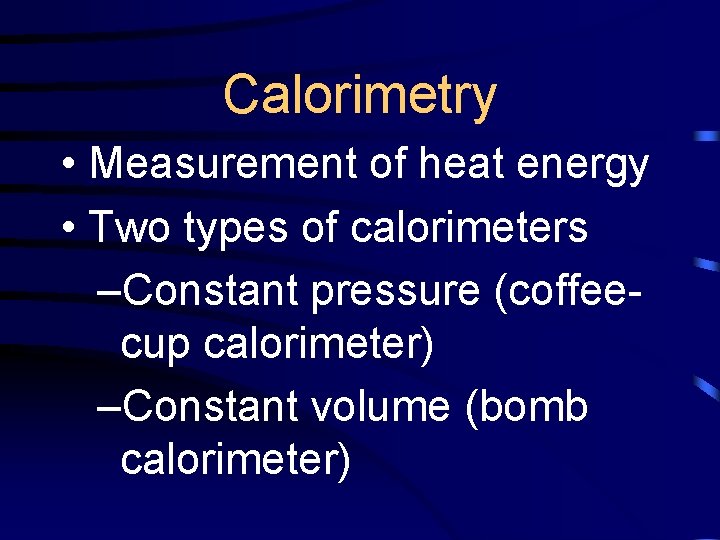
Calorimetry • Measurement of heat energy • Two types of calorimeters –Constant pressure (coffeecup calorimeter) –Constant volume (bomb calorimeter)

Biological Calorimetry • Nutrients –Carbohydrates –Proteins –Lipids –Water –Vitamins –minerals

Biological Calorimetry • Carbohydrates – 4 kcal/g or 17 k. J/g • Proteins – 4 kcal/g or 17 k. J/g • Lipids – 9 kcal/g or 38 k. J/g

Heat of Reaction • Hrxn • amount of heat absorbed or released in a chemical reaction • If absorbed, it is a reactant and the process is endothermic • If released, it is a product and the process is exothermic

Heat of Reaction • Deviations – Hformation is amount of heat absorbed or released during synthesis of one mole of an element or compound at 298 K and 1 atm of pressure

Heat of Reaction • Deviations – Hsolution is amount of heat absorbed or released when a substance dissolves in a solvent

Heat of Reaction • Deviations – Hcombustion is amount of heat released when a substance reacts with O 2 to form CO 2 and H 2 O

Heat of Reaction • Is part of the stoichiometry of a reaction…the heat of combustion of methane is 803 k. J CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) + 803 k. J • If there were 5 moles of CH 4 present, how many k. J would be produced?

Heat Energy Practice Problems 1. How many k. J are released by a reaction that raises the temperature of 1. 00 kg of water in a coffee-cup calorimeter from 25. 0°C to 27. 0°C? Psst…you know the SHC of water

2. A swimming pool measures 6. 0 m x 12. 0 m and is 3. 0 m deep all around. The pool is filled with water at a temperature of 20. 0°C. How many k. J must be released by the pool’s heater to raise the water temperature to 25. 0°C? Psst…the density of water is 1 g/cm 3, you know the SHC of water, and 1 m = 100 cm, so 1 m 3 would equal how many cm 3?

3. Gaseous butane, C 4 H 10, is burned in lots of lighters. Write the balanced equation for the complete combustion of butane. Butane’s heat of combustion is 2878 k. J. How many k. J of heat energy would be released by the combustion of 10. 0 g of butane?

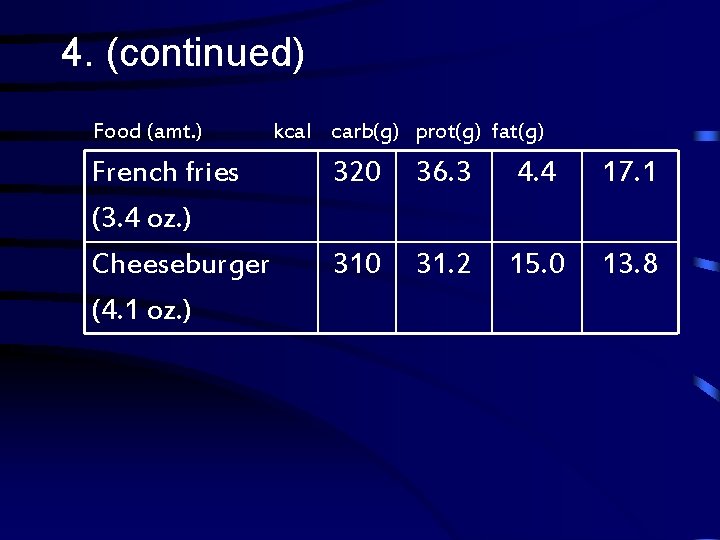
4. Use the table on the next slide to calculate the number of kilo. Joules provided by the fat in one serving of each of the following foods: a. french fries b. cheeseburger

4. (continued) Food (amt. ) French fries (3. 4 oz. ) Cheeseburger (4. 1 oz. ) kcal carb(g) prot(g) fat(g) 320 36. 3 4. 4 17. 1 310 31. 2 15. 0 13. 8

5. Is more energy released when 428 g of H 2 or 428 g of isooctane, C 8 H 18, react with an excess of oxygen? Psst…balance the equations. 2 H 2 + O 2 2 H 2 O + 484 k. J 2 C 8 H 18 + 25 O 2 16 CO 2 + 18 H 2 O + 4893 k. J

1. 2. 3. 4. 8. 36 k. J 4. 51 x 106 k. J 248 k. J a. 650 k. J b. 524 k. J 5. 428 g H 2 will release 5. 13 x 104 k. J while 428 g of C 8 H 18 will release 9. 16 x 103 k. J. So, the 428 g H 2 will release more energy.

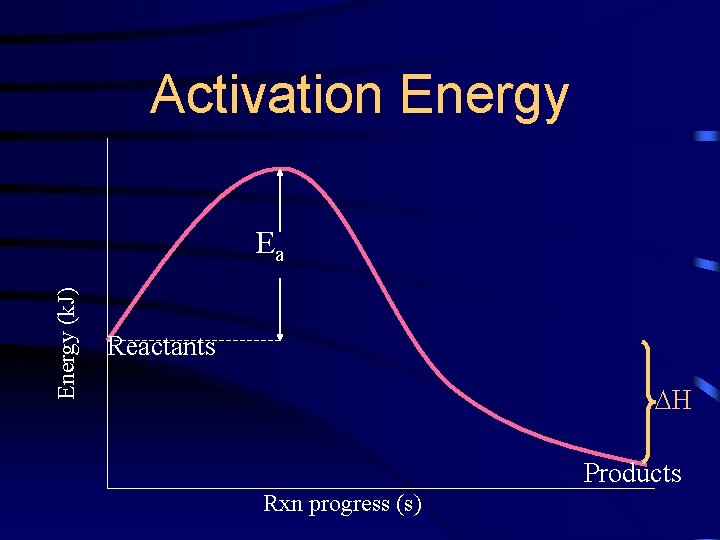
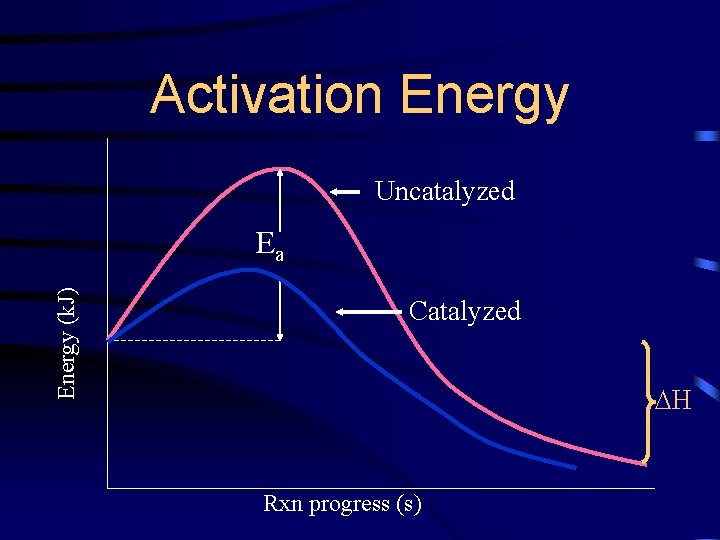
Activation Energy (k. J) Ea Reactants H Products Rxn progress (s)

Activation Energy Uncatalyzed Energy (k. J) Ea Catalyzed H Rxn progress (s)

Enthalpy • Enthalpy can be equated with heat energy • represented by H • H is also known as change in enthalpy

Hess’s Law • states that in going from a particular set of reactants to a particular set of products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps.

Finding H using Hess’s Law • If a reaction is reversed, the sign of H is also reversed. • If the coefficients in a balanced equation are multiplied by an integer, then the value of H is multiplied by that same integer.

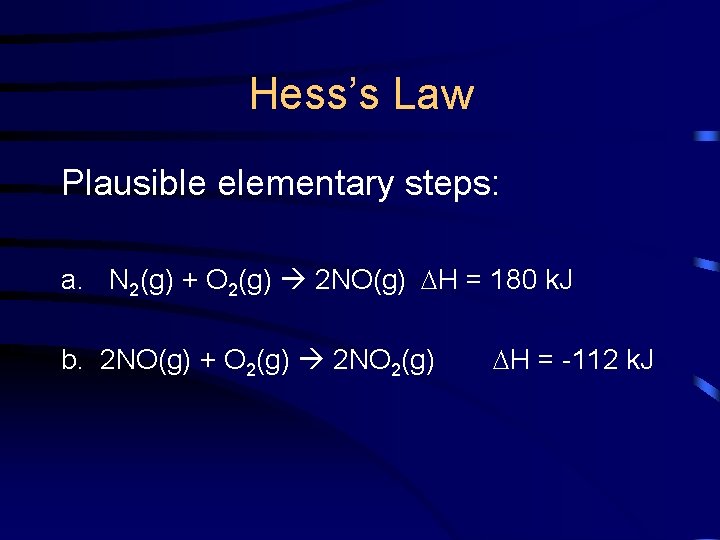
Hess’s Law • Consider the following reaction: N 2(g) + 2 O 2(g) 2 NO 2(g) • It does not necessarily occur as we see it. It can, in fact, occur in a few additive steps, known as elementary steps.

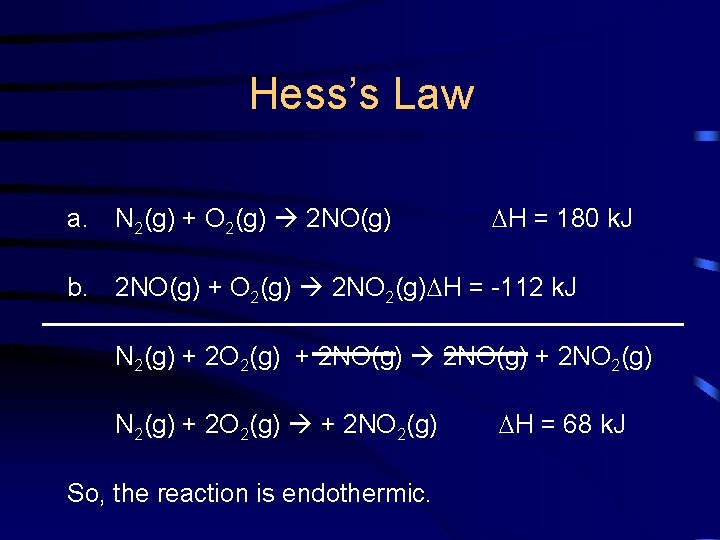
Hess’s Law Plausible elementary steps: a. N 2(g) + O 2(g) 2 NO(g) H = 180 k. J b. 2 NO(g) + O 2(g) 2 NO 2(g) H = -112 k. J

Hess’s Law a. N 2(g) + O 2(g) 2 NO(g) H = 180 k. J b. 2 NO(g) + O 2(g) 2 NO 2(g) H = -112 k. J N 2(g) + 2 O 2(g) + 2 NO(g) + 2 NO 2(g) N 2(g) + 2 O 2(g) + 2 NO 2(g) So, the reaction is endothermic. H = 68 k. J

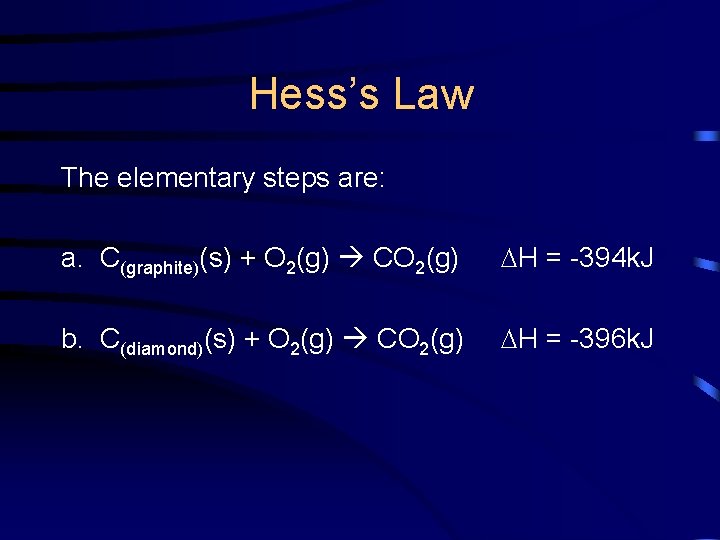
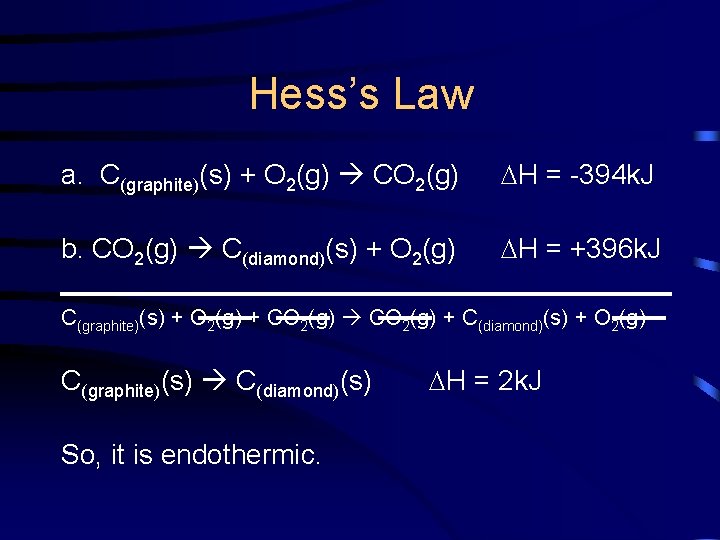
Hess’s Law Two forms of carbon are graphite and diamond. Using the enthalpies of combustion for graphite and diamond as your elementary steps, calculate the H for the conversion of graphite to diamond and state whether it is an endo- or exothermic process. C(graphite)(s) C(diamond)(s) H = ?

Hess’s Law The elementary steps are: a. C(graphite)(s) + O 2(g) CO 2(g) H = -394 k. J b. C(diamond)(s) + O 2(g) CO 2(g) H = -396 k. J

Hess’s Law a. C(graphite)(s) + O 2(g) CO 2(g) H = -394 k. J b. CO 2(g) C(diamond)(s) + O 2(g) H = +396 k. J C(graphite)(s) + O 2(g) + CO 2(g) + C(diamond)(s) + O 2(g) C(graphite)(s) C(diamond)(s) So, it is endothermic. H = 2 k. J

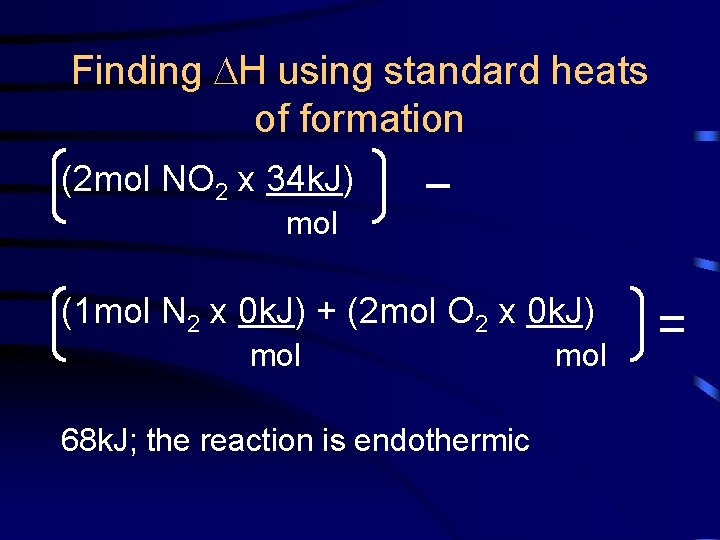
Finding H using standard heats of formation • H = ∑ Hf°products − ∑ Hf°reactants • Use pages A 21 -A 24 in the zumdahl Chemistry II textbook • All elements in their natural states will have Hf° equal to zero.

Finding H using standard heats of formation Find the H of the following reaction using Hf° values: N 2(g) + 2 O 2(g) 2 NO 2(g)

Finding H using standard heats of formation (2 mol NO 2 x 34 k. J) mol (1 mol N 2 x 0 k. J) + (2 mol O 2 x 0 k. J) mol 68 k. J; the reaction is endothermic mol

Finding H using standard heats of formation Find the H of the reaction which converts graphite to diamond using Hf° values.

Entropy • Is the measure of disorder or chaos present in a substance. • Chemical reactions may result in increasing disorder or decreasing disorder. • Represented by S…thus, change in entropy is S

Entropy • When there are moles of products than reactants, entropy usually increases. • When phase changes from more organized to less organized, entropy increases. • If S is positive, entropy increases; if negative, entropy decreases.

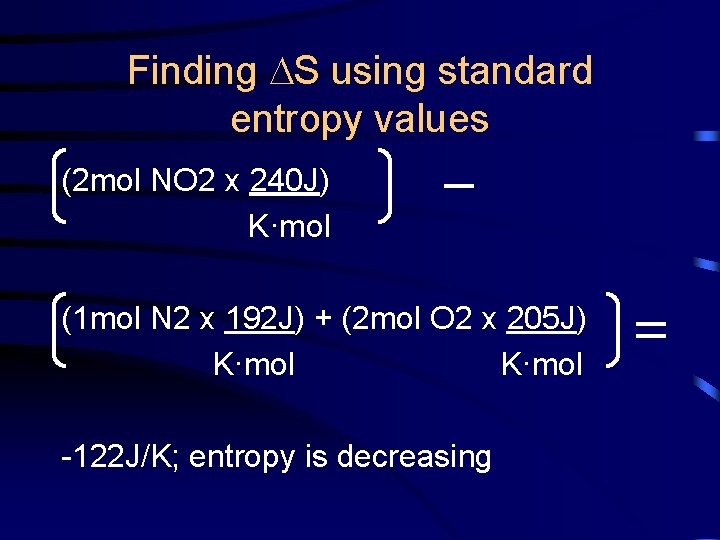
Finding S using standard entropy values • S = ∑ S°products − ∑ S°reactants • Use pages A 21 -A 24 in the zumdahl Chemistry II textbook

Finding S using standard entropy values Find the S of the following reaction using S° values: N 2(g) + 2 O 2(g) 2 NO 2(g)

Finding S using standard entropy values (2 mol NO 2 x 240 J) K·mol (1 mol N 2 x 192 J) + (2 mol O 2 x 205 J) K·mol -122 J/K; entropy is decreasing

Finding S using standard entropy values Find the S of the reaction which converts graphite to diamond using S° values.

Spontaneity refers to whether a reaction will happen without outside intervention or not. It says nothing about how quickly the reaction will happen only that it will or will not occur.

Free Energy • is symbolized by G and is used to determine the spontaneity of a reaction • G = H T S

Free Energy • If G is positive, it is a nonspontaneous process and is known as an endergonic reaction. • If G is negative, it is a spontaneous process and is known as an exergonic reaction.

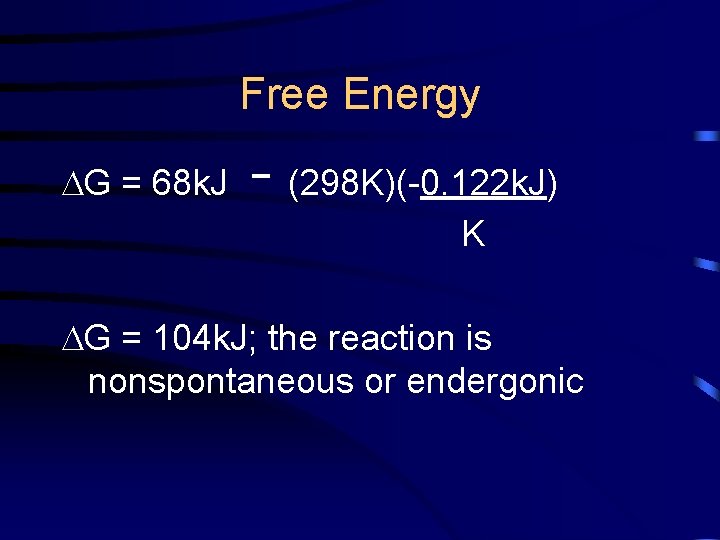
Free Energy Find the G of the following reaction at 25°C: N 2(g) + 2 O 2(g) 2 NO 2(g)

Free Energy G = 68 k. J (298 K)(-0. 122 k. J) K G = 104 k. J; the reaction is nonspontaneous or endergonic

Free Energy Find the G of the reaction which converts graphite to diamond at 100°C and state whether it is spontaneous or not.

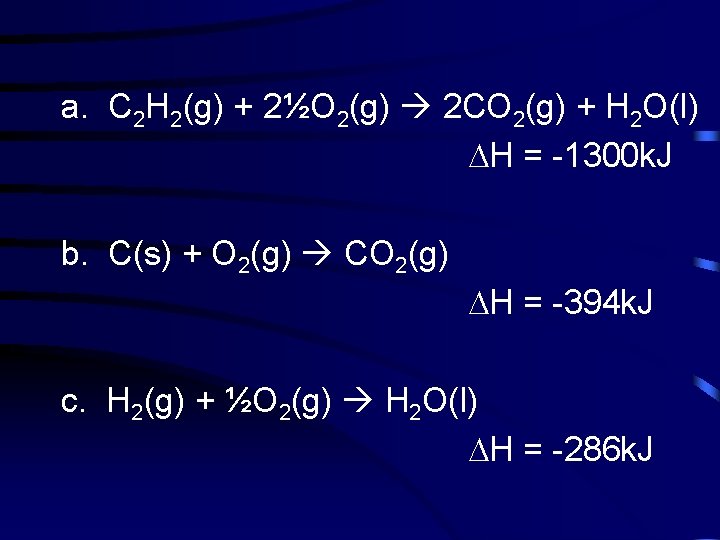
More Practice Problems… 6. Acetylene gas, C 2 H 2, is used in some welding applications and can be made via the following reaction: 2 C(s) + H 2(g) C 2 H 2(g) Determine its H using the elementary steps on the following slide.

a. C 2 H 2(g) + 2½O 2(g) 2 CO 2(g) + H 2 O(l) H = -1300 k. J b. C(s) + O 2(g) CO 2(g) H = -394 k. J c. H 2(g) + ½O 2(g) H 2 O(l) H = -286 k. J

7. Think about photosynthesis… recall that carbon dioxide gas reacts with water to produce solid glucose (C 6 H 12 O 6) and oxygen gas. Write a balanced equation, and determine the H, S, and G at 25°C. State whether the reaction is endo- or exothermic, whether entropy increases or decreases, and whether it spontaneous or not.

6. H = +226 k. J 7. H = +2802 k. J; endothermic S = -262 J/K; entropy decreases G = +2880 k. J; nonspontaneous