Thermodynamics Chapter 19 Important vocabulary to review Heat

Thermodynamics Chapter 19

Important vocabulary to review: • • Heat Temperature Energy State function/property System Surroundings Work Driving force
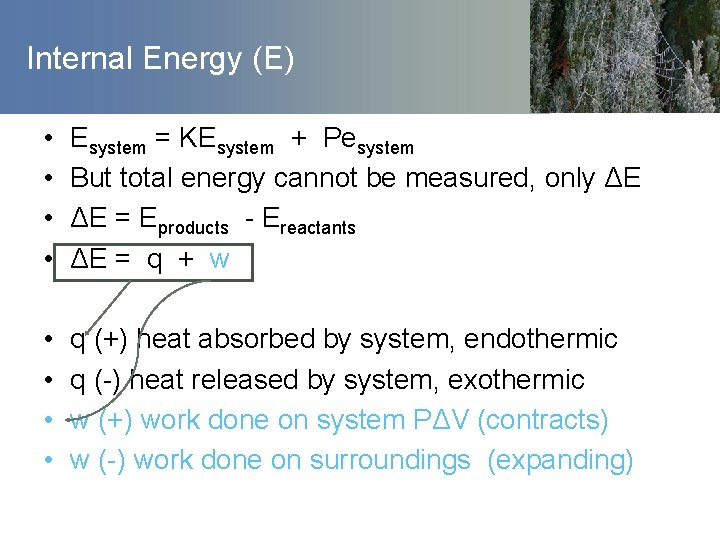
Internal Energy (E) • • Esystem = KEsystem + Pesystem But total energy cannot be measured, only ΔE ΔE = Eproducts - Ereactants ΔE = q + w • • q (+) heat absorbed by system, endothermic q (-) heat released by system, exothermic w (+) work done on system PΔV (contracts) w (-) work done on surroundings (expanding)
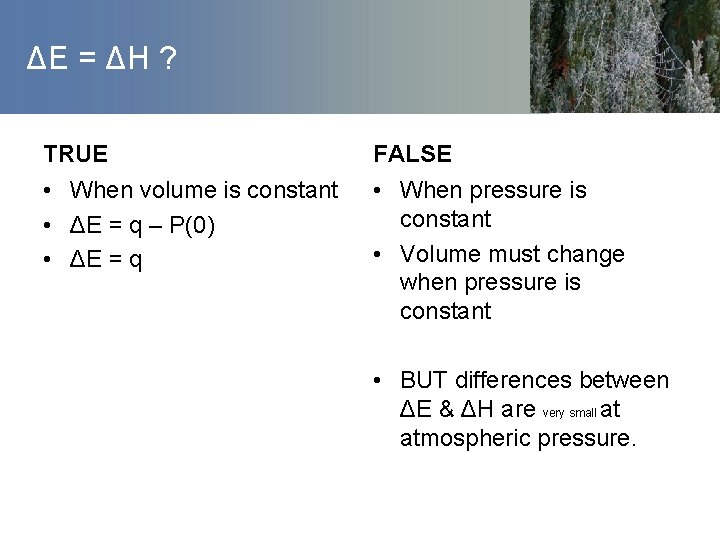
ΔE = ΔH ? TRUE FALSE • When volume is constant • ΔE = q – P(0) • ΔE = q • When pressure is constant • Volume must change when pressure is constant • BUT differences between ΔE & ΔH are very small at atmospheric pressure.

First Law of Thermodynamics • The energy of the universe is constant • Heat lost = heat gained • Law of conservation of energy

Spontaneous Processes • Occurs without outside intervention • Variable speed • Everything that happens begins with a spontaneous change somewhere!
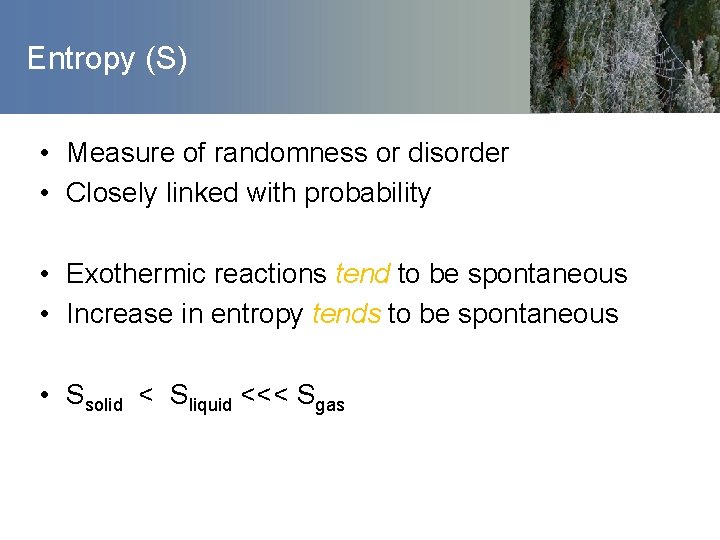
Entropy (S) • Measure of randomness or disorder • Closely linked with probability • Exothermic reactions tend to be spontaneous • Increase in entropy tends to be spontaneous • Ssolid < Sliquid <<< Sgas
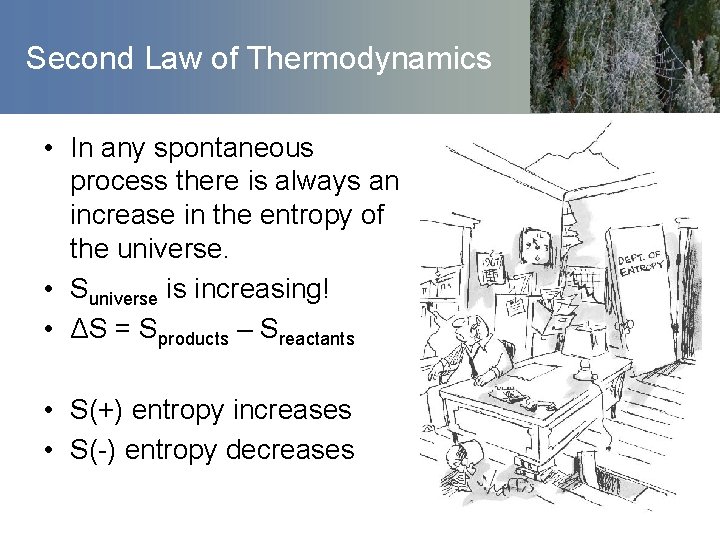
Second Law of Thermodynamics • In any spontaneous process there is always an increase in the entropy of the universe. • Suniverse is increasing! • ΔS = Sproducts – Sreactants • S(+) entropy increases • S(-) entropy decreases
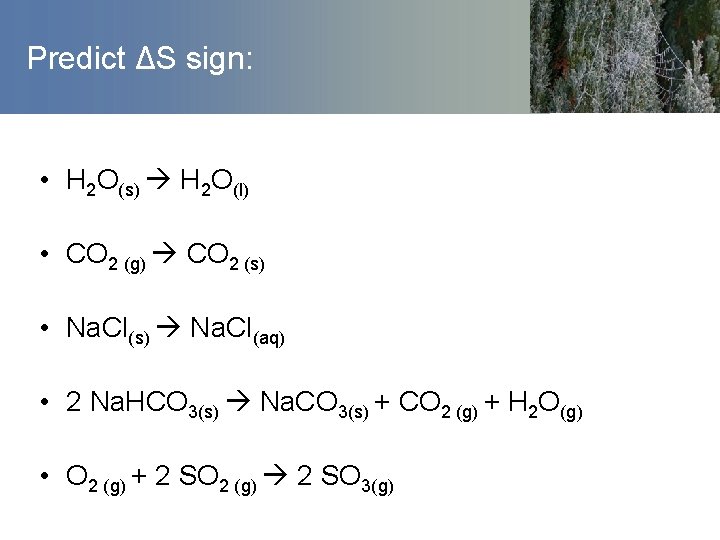
Predict ΔS sign: • H 2 O(s) H 2 O(l) • CO 2 (g) CO 2 (s) • Na. Cl(s) Na. Cl(aq) • 2 Na. HCO 3(s) Na. CO 3(s) + CO 2 (g) + H 2 O(g) • O 2 (g) + 2 SO 2 (g) 2 SO 3(g)
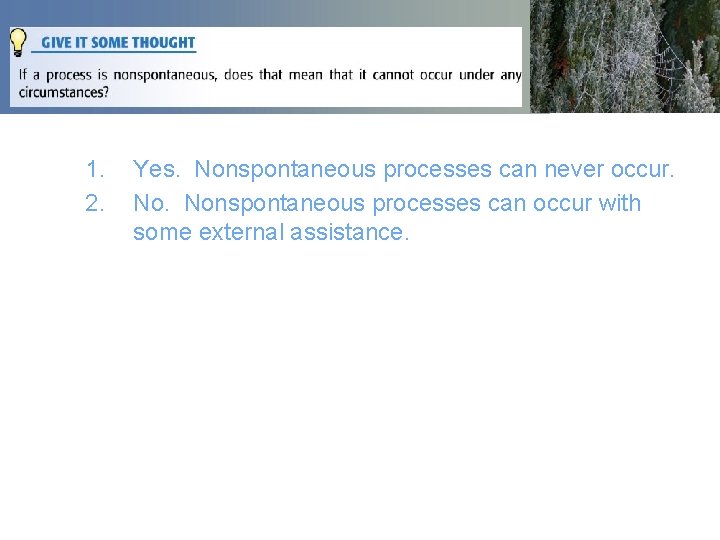
1. 2. Yes. Nonspontaneous processes can never occur. Nonspontaneous processes can occur with some external assistance.
- Slides: 10