Thermodynamics Chapter 18 Free Energy and Temperature Free
- Slides: 14

Thermodynamics Chapter 18

Free Energy and Temperature

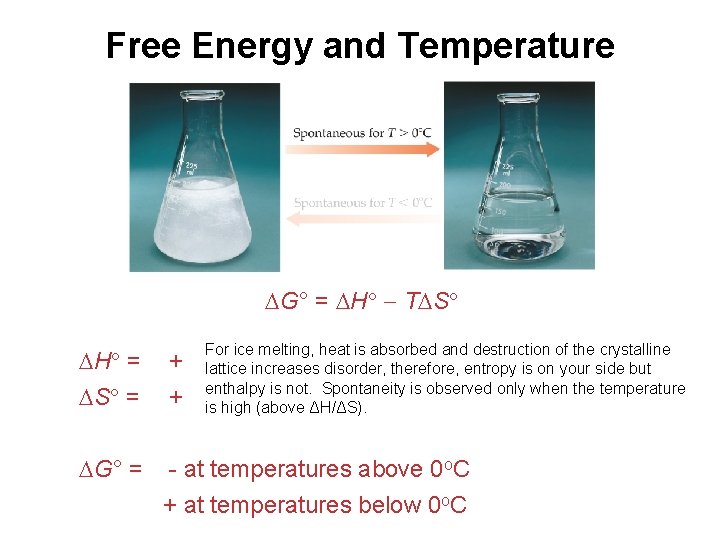
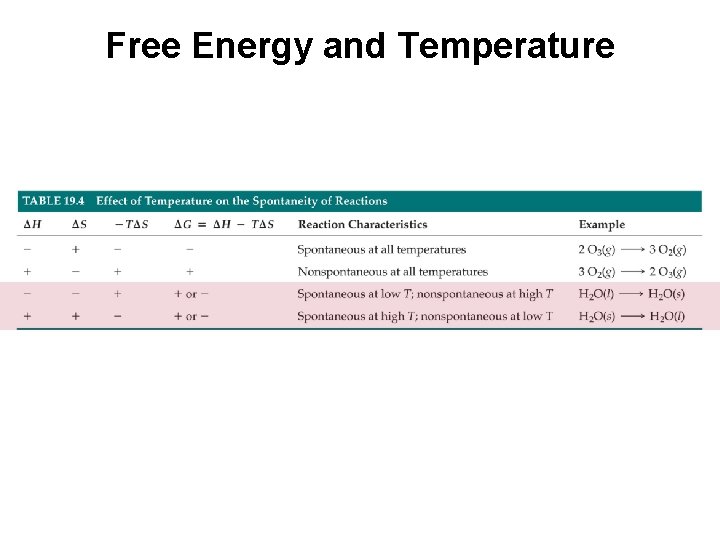
Free Energy and Temperature G° = H T S For ice melting, heat is absorbed and destruction of the crystalline lattice increases disorder, therefore, entropy is on your side but enthalpy is not. Spontaneity is observed only when the temperature is high (above ΔH/ΔS). H = S = + + G° = - at temperatures above 0 o. C + at temperatures below 0 o. C

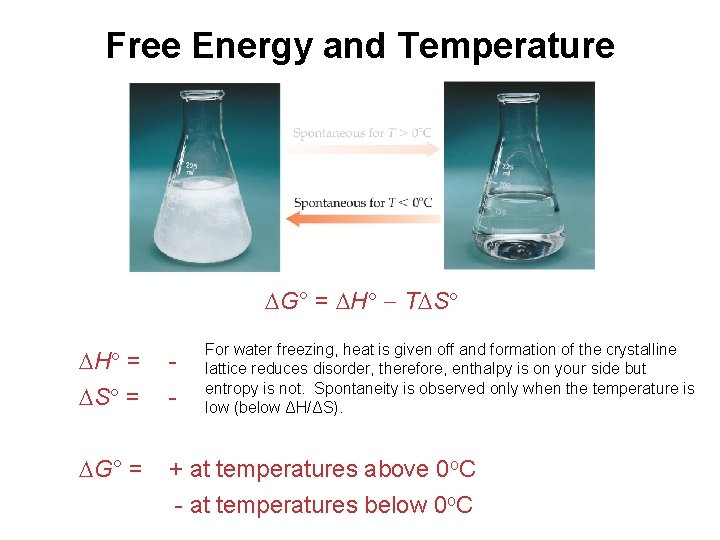
Free Energy and Temperature G° = H T S For water freezing, heat is given off and formation of the crystalline lattice reduces disorder, therefore, enthalpy is on your side but entropy is not. Spontaneity is observed only when the temperature is low (below ΔH/ΔS). H = S = - G° = + at temperatures above 0 o. C - at temperatures below 0 o. C

Free Energy and Temperature

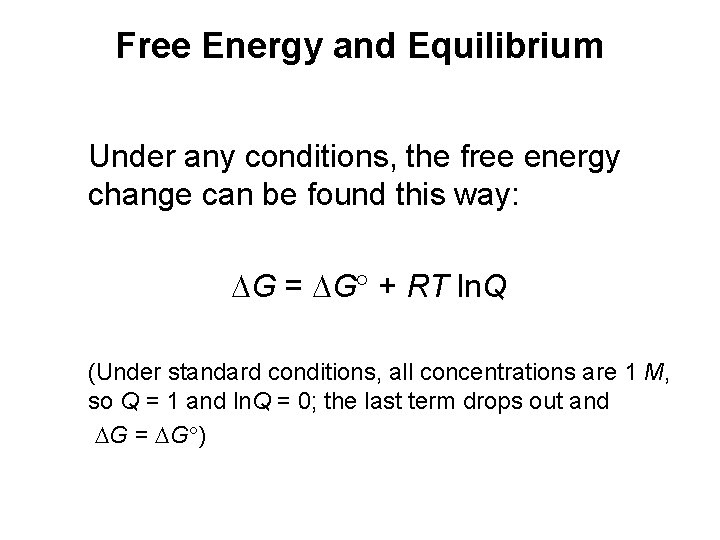
Free Energy and Equilibrium Under any conditions, the free energy change can be found this way: G = G + RT ln. Q (Under standard conditions, all concentrations are 1 M, so Q = 1 and ln. Q = 0; the last term drops out and G = G )

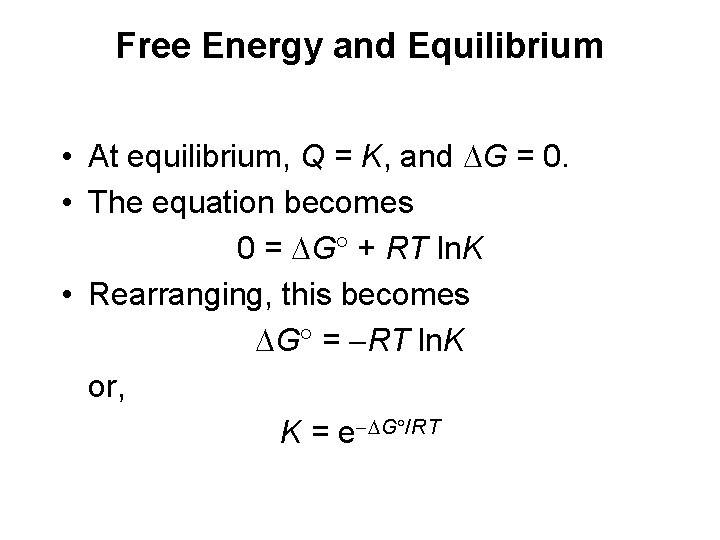
Free Energy and Equilibrium • At equilibrium, Q = K, and G = 0. • The equation becomes 0 = G + RT ln. K • Rearranging, this becomes G = RT ln. K or, K = e G /RT

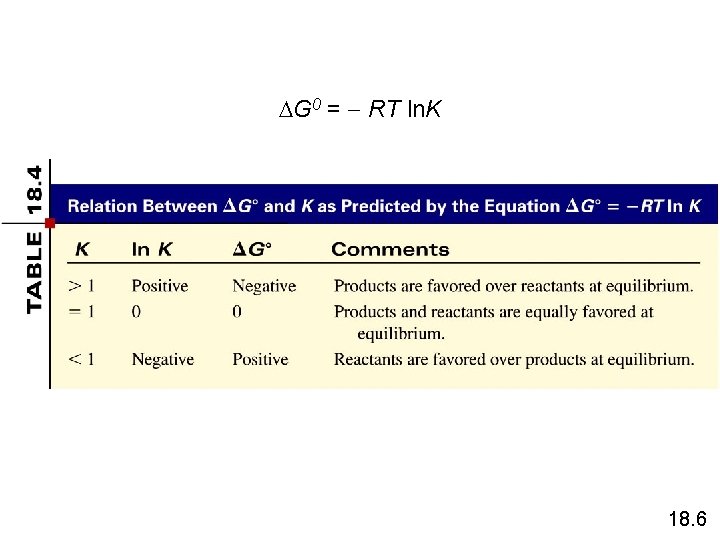
G 0 = RT ln. K 18. 6

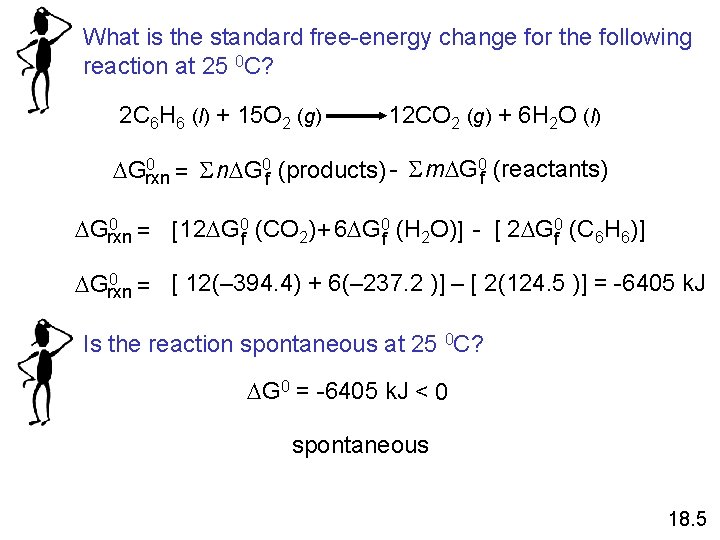
What is the standard free-energy change for the following reaction at 25 0 C? 2 C 6 H 6 (l) + 15 O 2 (g) 12 CO 2 (g) + 6 H 2 O (l) 0 Grxn = S n G 0 f (products) - S m G 0 f (reactants) 0 Grxn = [12 G 0 f (CO 2) + 6 G 0 f (H 2 O)] - [ 2 G 0 f (C 6 H 6)] 0 Grxn = [ 12(– 394. 4) + 6(– 237. 2 )] – [ 2(124. 5 )] = -6405 k. J Is the reaction spontaneous at 25 0 C? G 0 = -6405 k. J < 0 spontaneous 18. 5

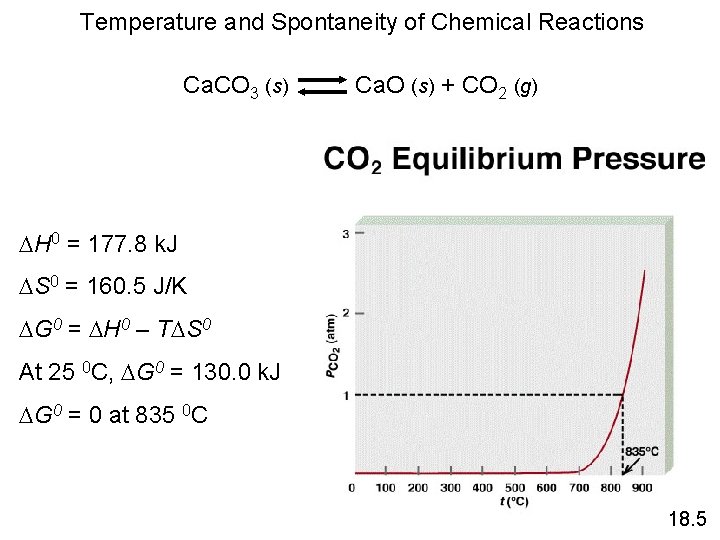
Temperature and Spontaneity of Chemical Reactions Ca. CO 3 (s) Ca. O (s) + CO 2 (g) H 0 = 177. 8 k. J S 0 = 160. 5 J/K G 0 = H 0 – T S 0 At 25 0 C, G 0 = 130. 0 k. J G 0 = 0 at 835 0 C 18. 5

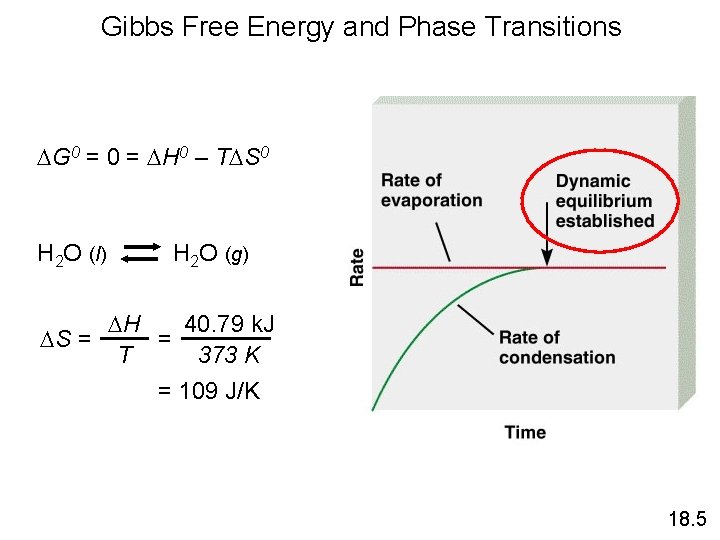
Gibbs Free Energy and Phase Transitions G 0 = H 0 – T S 0 H 2 O (l) H 2 O (g) H 40. 79 k. J S = = 373 K T = 109 J/K 18. 5

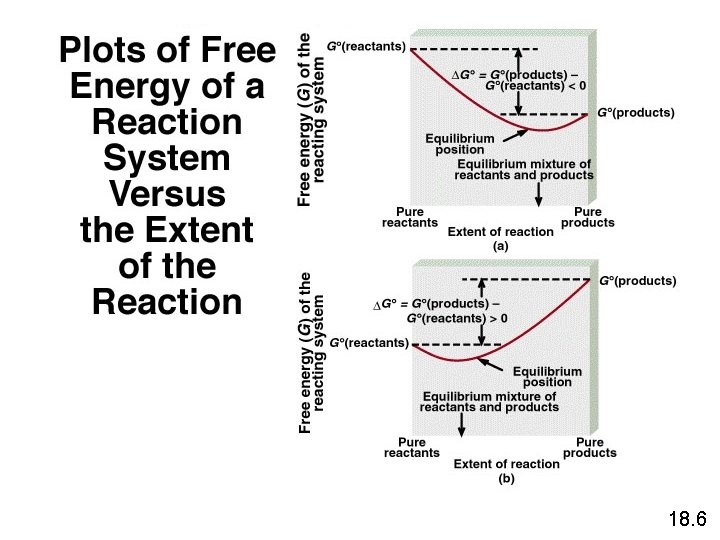
18. 6

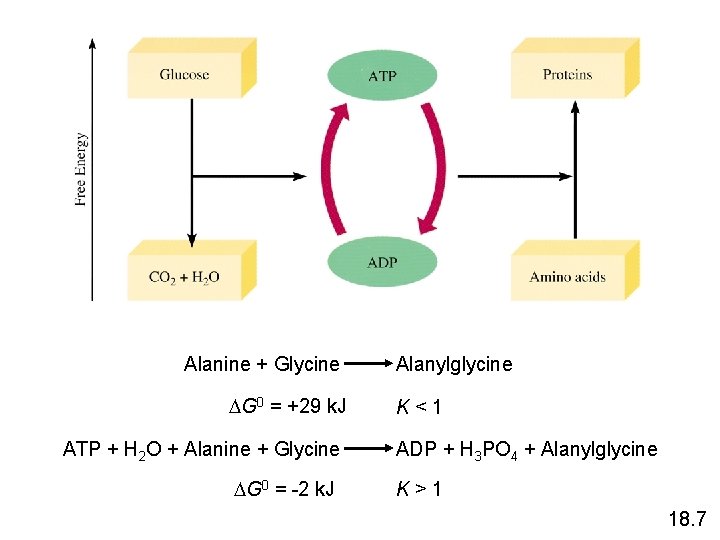
Alanine + Glycine G 0 = +29 k. J ATP + H 2 O + Alanine + Glycine G 0 = -2 k. J Alanylglycine K<1 ADP + H 3 PO 4 + Alanylglycine K>1 18. 7

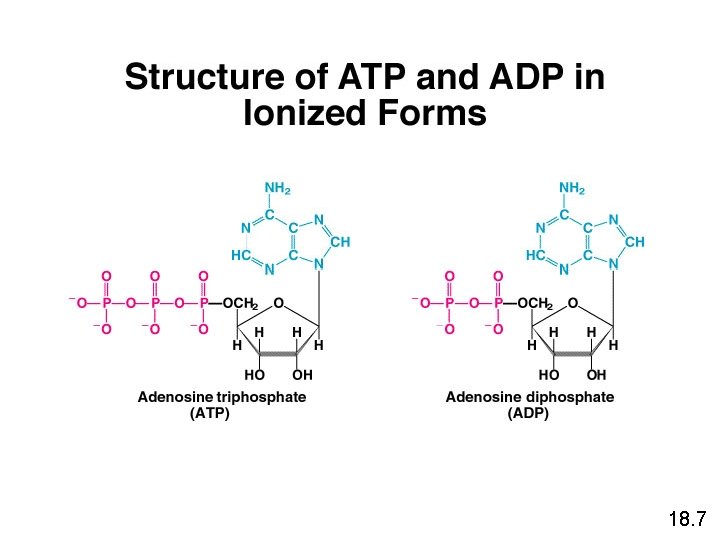
18. 7