Thermodynamics and Statistical Mechanics Thermodynamic Potentials Thermo Stat






- Slides: 19

Thermodynamics and Statistical Mechanics Thermodynamic Potentials Thermo & Stat Mech Spring 2006 Class 8 1

Thermodynamic Potentials There are two energy functions that have been used so far: Internal Energy Enthalpy There are two more. Thermo & Stat Mech - Spring 2006 Class 8 2

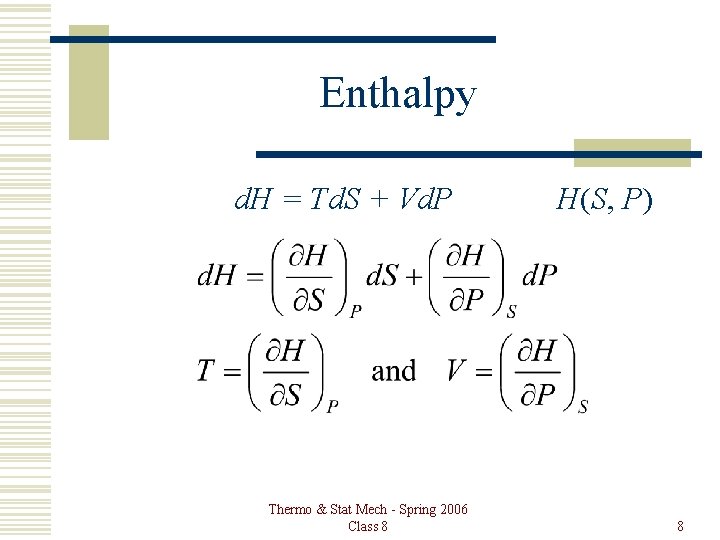
Properties From first law: Td. S = d. U + Pd. V, or Internal Energy d. U = Td. S – Pd. V U(S, V) Enthalpy: H = U + PV d. H = Td. S + Vd. P Thermo & Stat Mech - Spring 2006 Class 8 H(S, P) 3

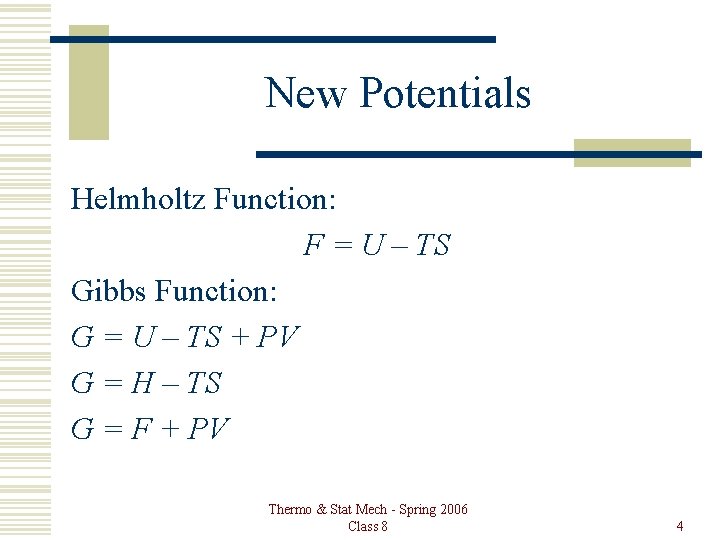
New Potentials Helmholtz Function: F = U – TS Gibbs Function: G = U – TS + PV G = H – TS G = F + PV Thermo & Stat Mech - Spring 2006 Class 8 4

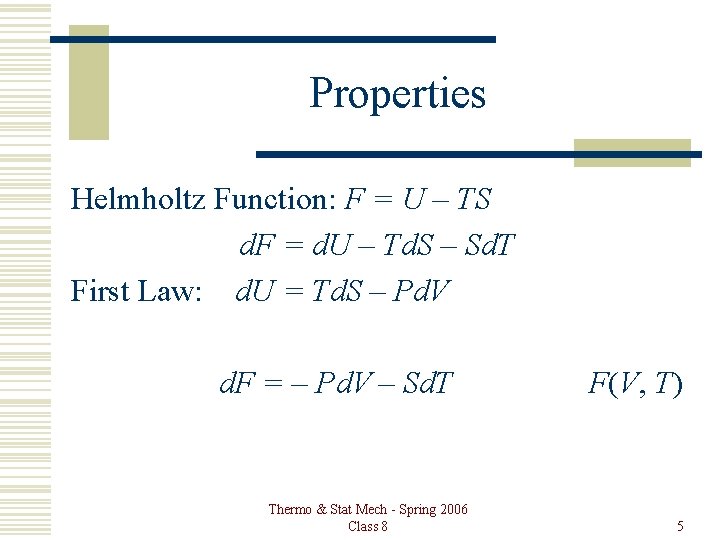
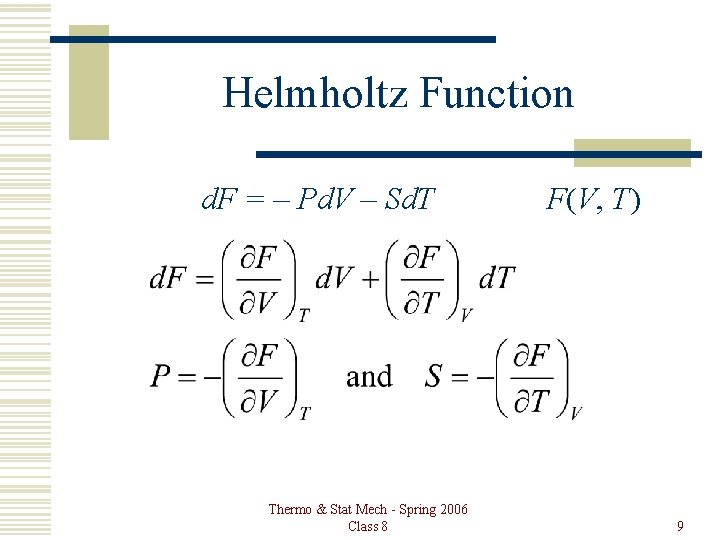
Properties Helmholtz Function: F = U – TS d. F = d. U – Td. S – Sd. T First Law: d. U = Td. S – Pd. V d. F = – Pd. V – Sd. T Thermo & Stat Mech - Spring 2006 Class 8 F(V, T) 5

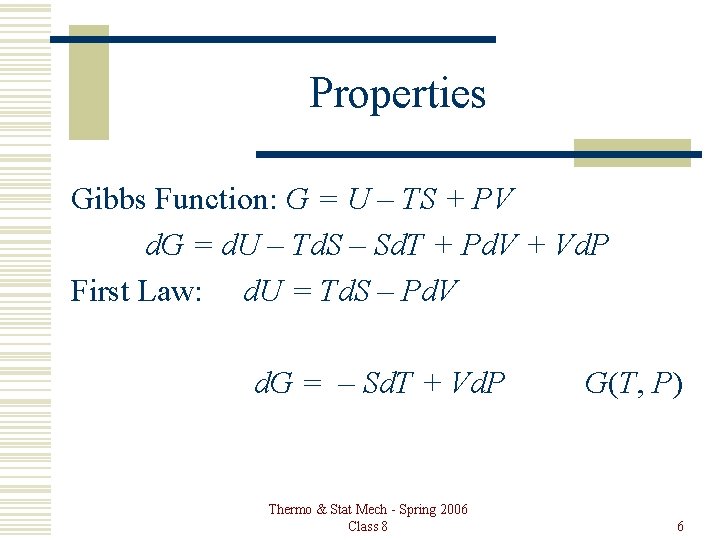
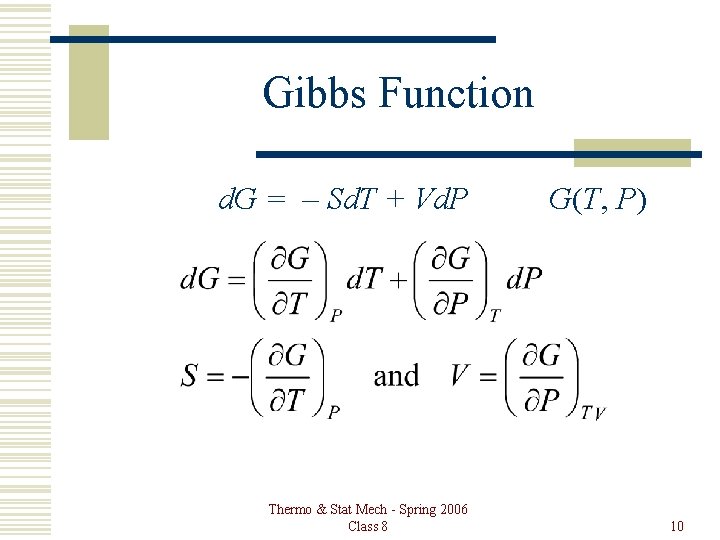
Properties Gibbs Function: G = U – TS + PV d. G = d. U – Td. S – Sd. T + Pd. V + Vd. P First Law: d. U = Td. S – Pd. V d. G = – Sd. T + Vd. P Thermo & Stat Mech - Spring 2006 Class 8 G(T, P) 6

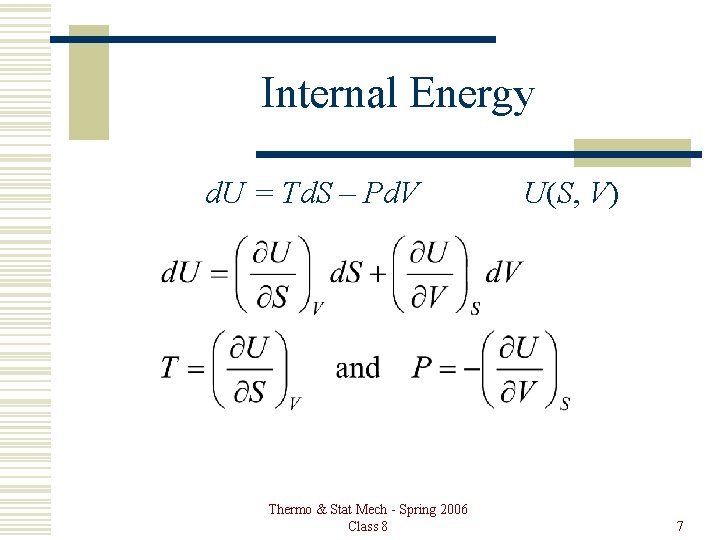
Internal Energy d. U = Td. S – Pd. V Thermo & Stat Mech - Spring 2006 Class 8 U(S, V) 7

Enthalpy d. H = Td. S + Vd. P Thermo & Stat Mech - Spring 2006 Class 8 H(S, P) 8

Helmholtz Function d. F = – Pd. V – Sd. T Thermo & Stat Mech - Spring 2006 Class 8 F(V, T) 9

Gibbs Function d. G = – Sd. T + Vd. P Thermo & Stat Mech - Spring 2006 Class 8 G(T, P) 10

All Four d. U = Td. S – Pd. V d. H = Td. S + Vd. P d. F = – Pd. V – Sd. T d. G = – Sd. T + Vd. P Thermo & Stat Mech - Spring 2006 Class 8 U(S, V) H(S, P) F(V, T) G(T, P) 11

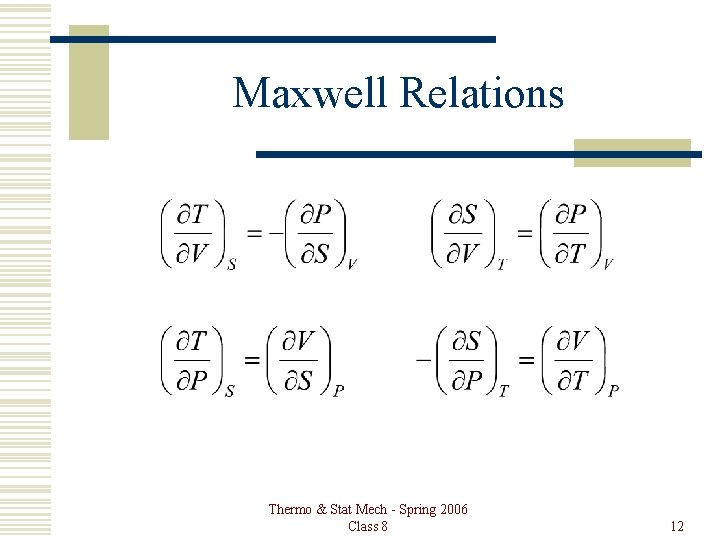
Maxwell Relations Thermo & Stat Mech - Spring 2006 Class 8 12

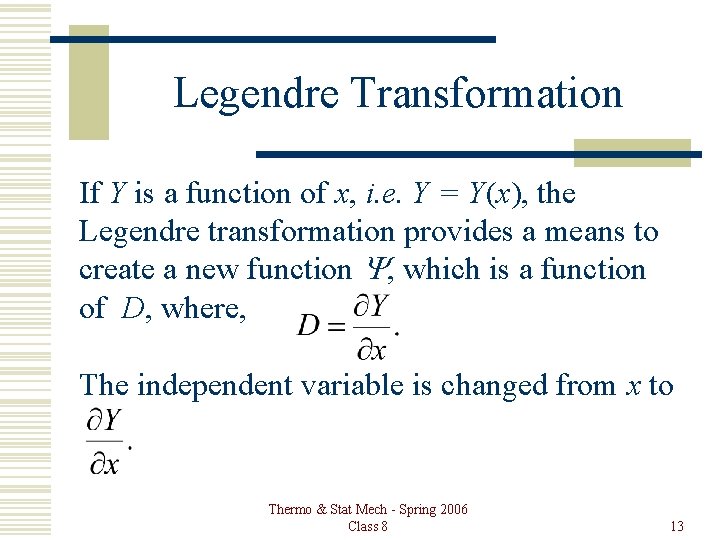
Legendre Transformation If Y is a function of x, i. e. Y = Y(x), the Legendre transformation provides a means to create a new function Y, which is a function of D, where, The independent variable is changed from x to Thermo & Stat Mech - Spring 2006 Class 8 13

Legendre Transformation Thermo & Stat Mech - Spring 2006 Class 8 14

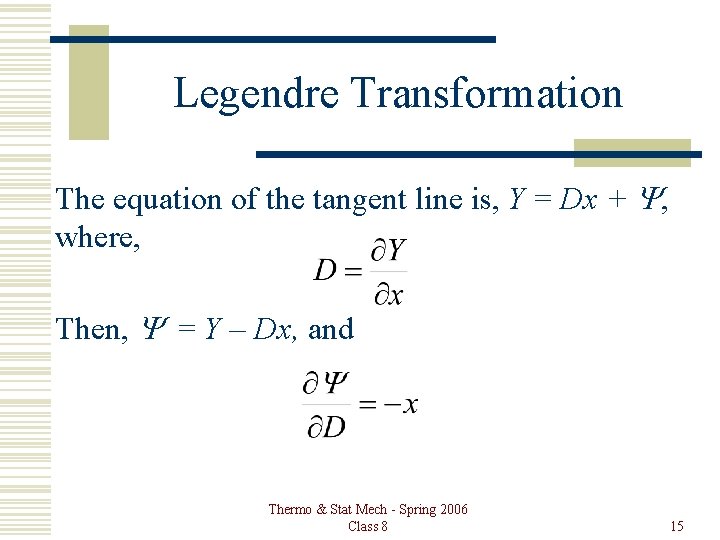
Legendre Transformation The equation of the tangent line is, Y = Dx + Y, where, Then, Y = Y – Dx, and Thermo & Stat Mech - Spring 2006 Class 8 15

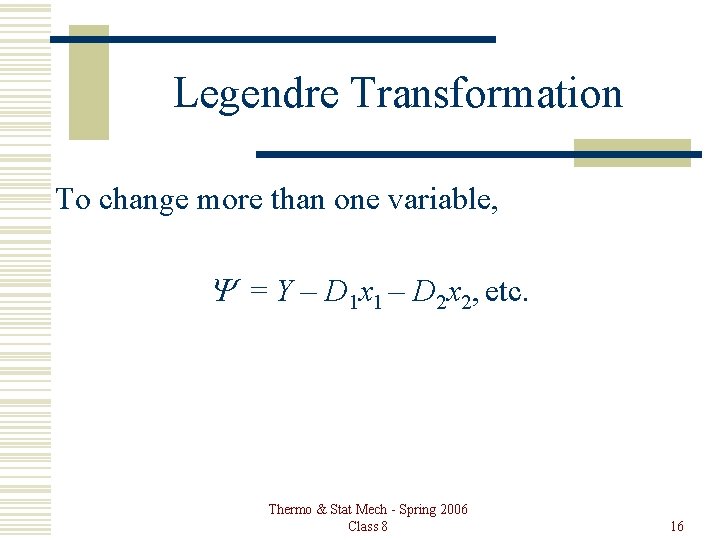
Legendre Transformation To change more than one variable, Y = Y – D 1 x 1 – D 2 x 2, etc. Thermo & Stat Mech - Spring 2006 Class 8 16

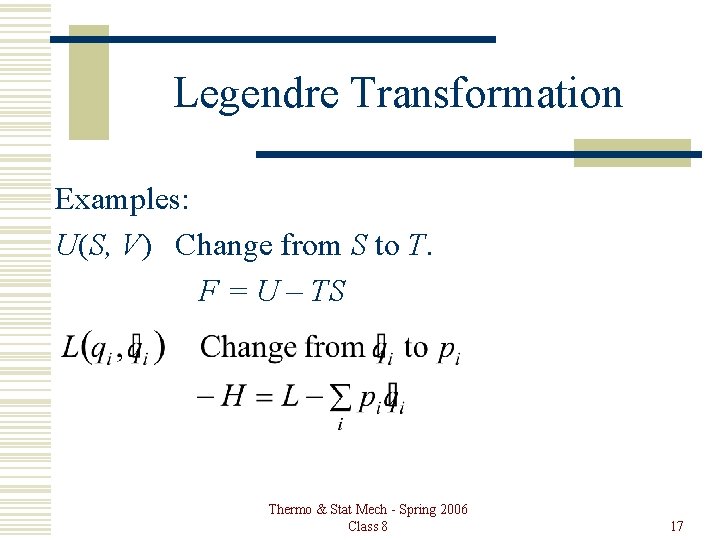
Legendre Transformation Examples: U(S, V) Change from S to T. F = U – TS Thermo & Stat Mech - Spring 2006 Class 8 17

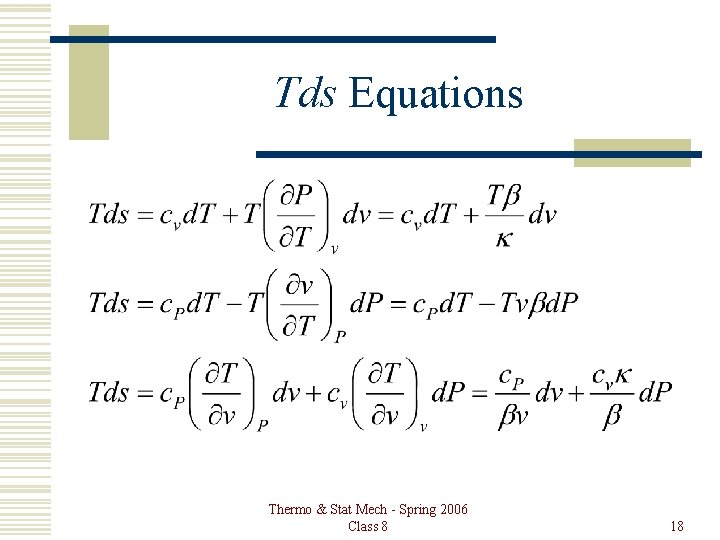
Tds Equations Thermo & Stat Mech - Spring 2006 Class 8 18

Joule-Thomson coefficient h = u+Pv dh = đq + vdp = Tds + vdp = 0 Tds = – vd. P Thermo & Stat Mech - Spring 2006 Class 8 19