Thermodynamics and Statistical Mechanics Review for Quiz 1











- Slides: 25

Thermodynamics and Statistical Mechanics Review for Quiz 1 Thermo & Stat Mech Spring 2006 Class 11

Laws of Thermodynamics First law: đQ – đW = d. U Energy is conserved Thermo & Stat Mech - Spring 2006 Class 11 2

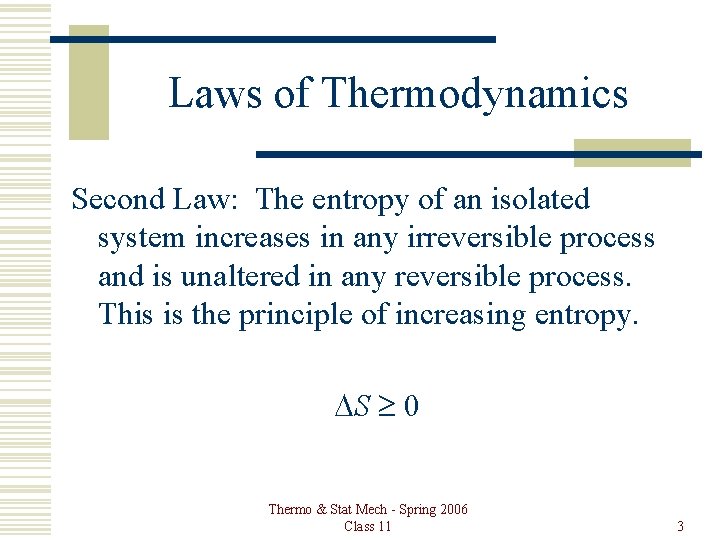
Laws of Thermodynamics Second Law: The entropy of an isolated system increases in any irreversible process and is unaltered in any reversible process. This is the principle of increasing entropy. DS ³ 0 Thermo & Stat Mech - Spring 2006 Class 11 3

Laws of Thermodynamics Third Law: The entropy of a true equilibrium state of a system at a temperature of absolute zero is zero. Equivalent to: It is impossible to reduce the temperature of a system to absolute zero using a finite number of processes. Thermo & Stat Mech - Spring 2006 Class 11 4

Second Law Variations No series of processes is possible whose sole result is the absorption of heat from a thermal reservoir and the complete conversion of this energy to work. There are no perfect engines! Thermo & Stat Mech - Spring 2006 Class 11 5

Second Law Variations No series of processes is possible whose sole result is the transfer of heat from a reservoir at a given temperature to a reservoir at a higher temperature. There are no perfect refrigerators! Thermo & Stat Mech - Spring 2006 Class 11 6

Zeroth Law If two systems are separately in thermal equilibrium with a third system, they are in thermal equilibrium with each other. Thermo & Stat Mech - Spring 2006 Class 11 7

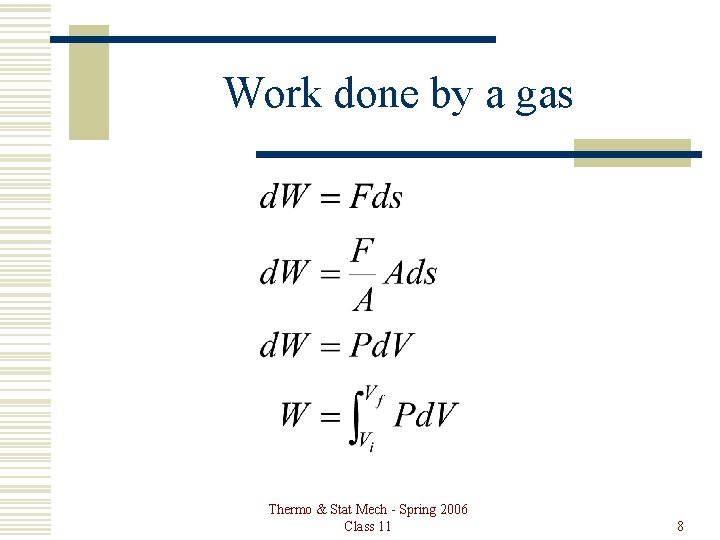
Work done by a gas Thermo & Stat Mech - Spring 2006 Class 11 8

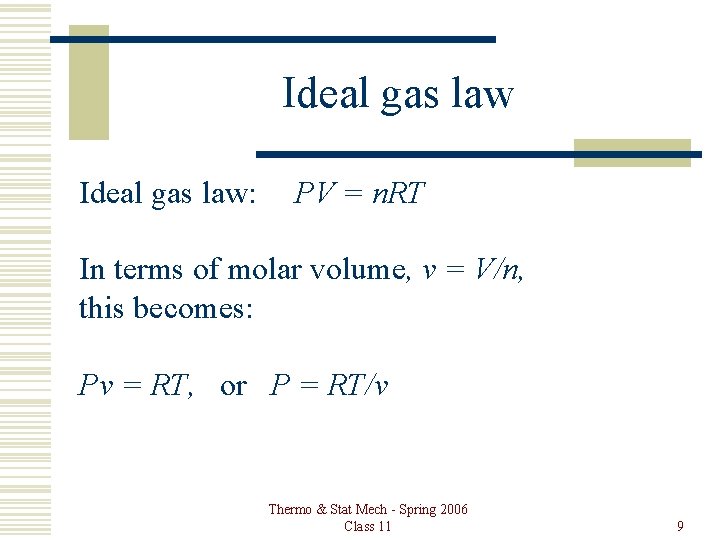
Ideal gas law: PV = n. RT In terms of molar volume, v = V/n, this becomes: Pv = RT, or P = RT/v Thermo & Stat Mech - Spring 2006 Class 11 9

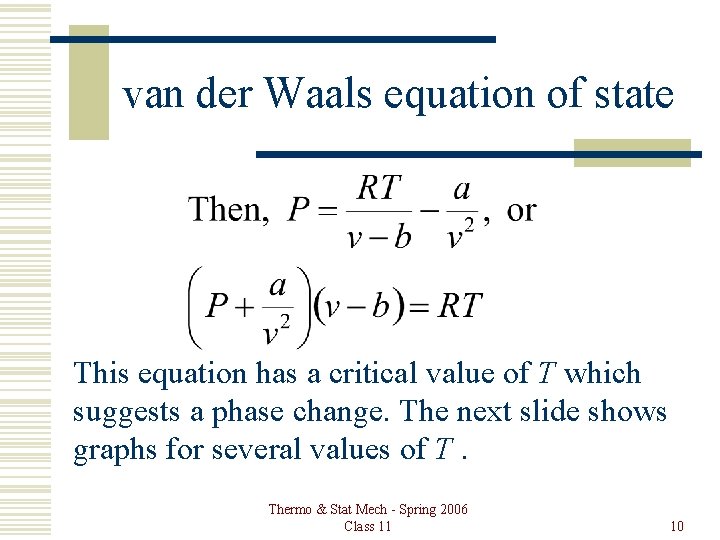
van der Waals equation of state This equation has a critical value of T which suggests a phase change. The next slide shows graphs for several values of T. Thermo & Stat Mech - Spring 2006 Class 11 10

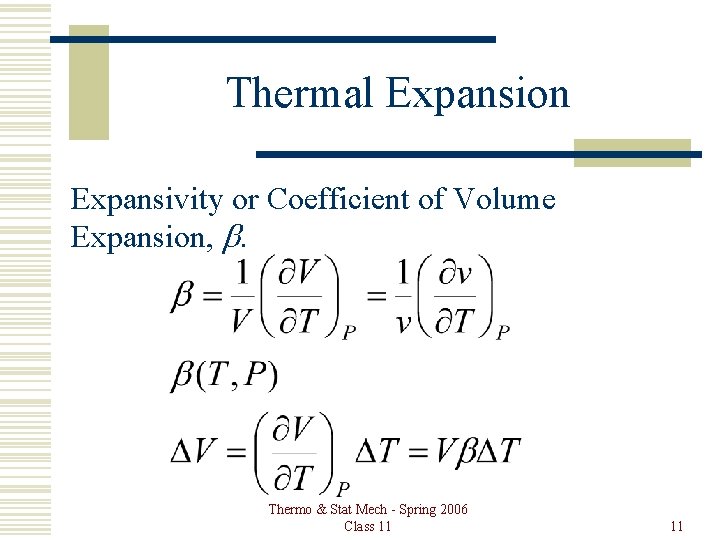
Thermal Expansion Expansivity or Coefficient of Volume Expansion, b. Thermo & Stat Mech - Spring 2006 Class 11 11

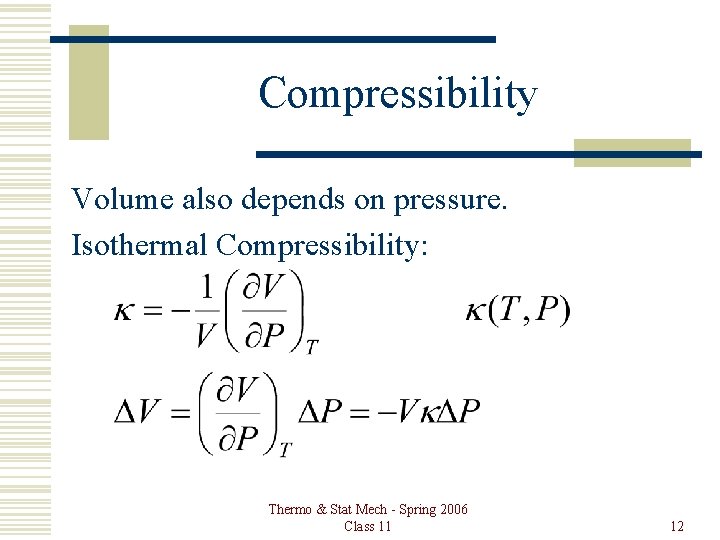
Compressibility Volume also depends on pressure. Isothermal Compressibility: Thermo & Stat Mech - Spring 2006 Class 11 12

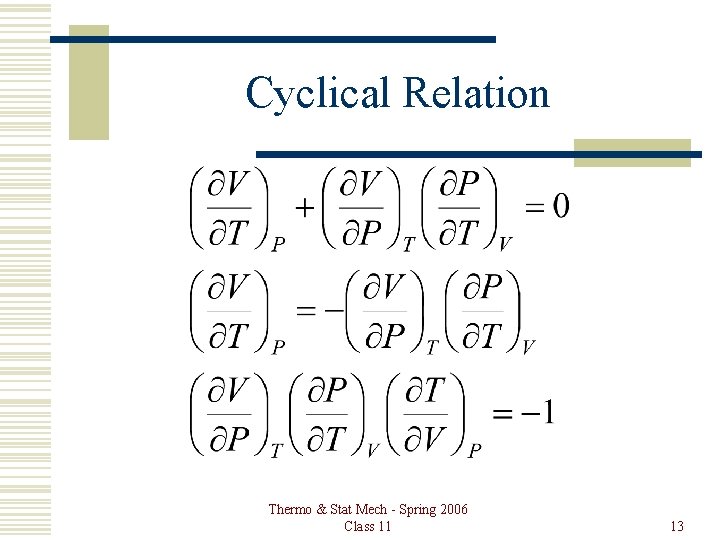
Cyclical Relation Thermo & Stat Mech - Spring 2006 Class 11 13

Carnot Cycle A Carnot cycle is an idealized reversible cycle that operates between two heat reservoirs at temperatures T 1 and T 2, where T 2 > T 1. It can operate as a heat engine, or a refrigerator. Thermo & Stat Mech - Spring 2006 Class 11 14

Thermal Efficiency (h) If T 1 = 0, h = 1 (100%) Thermo & Stat Mech - Spring 2006 Class 11 15

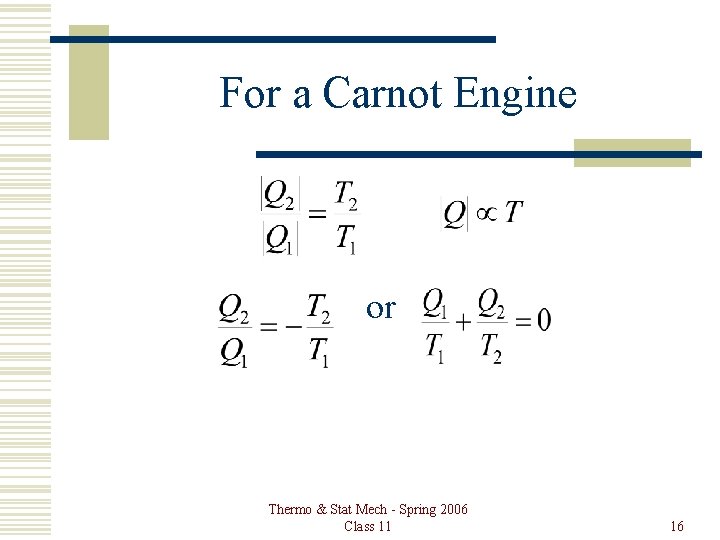
For a Carnot Engine or Thermo & Stat Mech - Spring 2006 Class 11 16

Entropy For reversible processes. Entropy is a state variable. Thermo & Stat Mech - Spring 2006 Class 11 17

First and Second Laws First Law: d. U = đQ – đW First law, combined with the second law: d. U = Td. S – Pd. V Thermo & Stat Mech - Spring 2006 Class 11 18

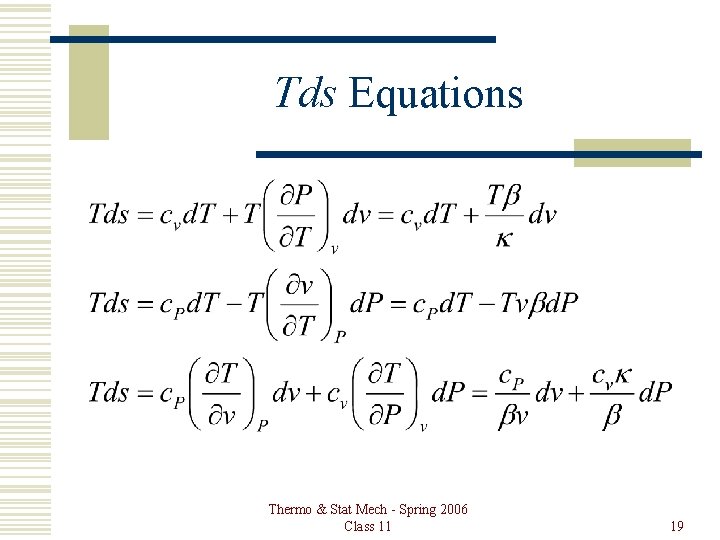
Tds Equations Thermo & Stat Mech - Spring 2006 Class 11 19

Ideal Gas Thermo & Stat Mech - Spring 2006 Class 11 20

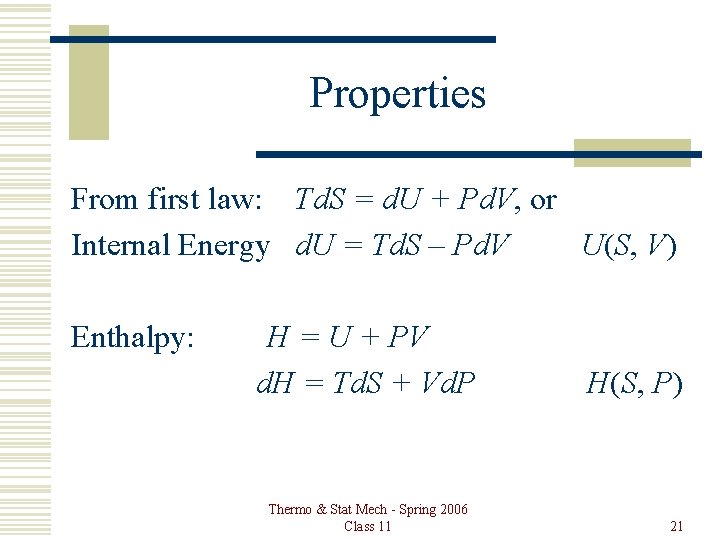
Properties From first law: Td. S = d. U + Pd. V, or Internal Energy d. U = Td. S – Pd. V U(S, V) Enthalpy: H = U + PV d. H = Td. S + Vd. P Thermo & Stat Mech - Spring 2006 Class 11 H(S, P) 21

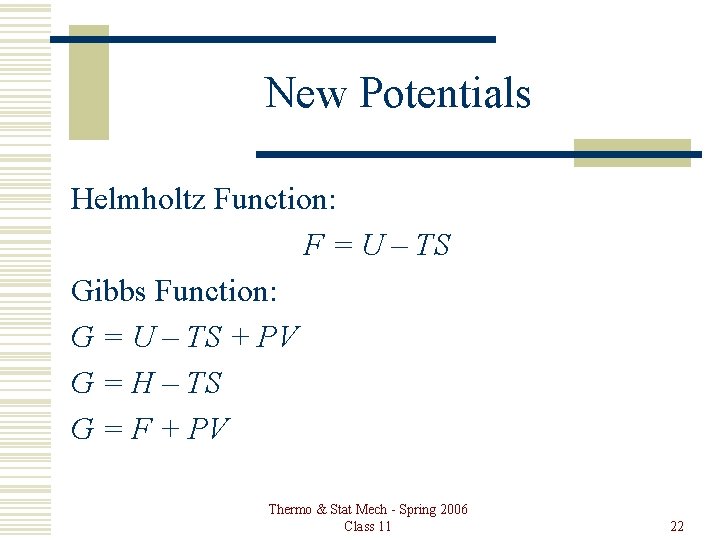
New Potentials Helmholtz Function: F = U – TS Gibbs Function: G = U – TS + PV G = H – TS G = F + PV Thermo & Stat Mech - Spring 2006 Class 11 22

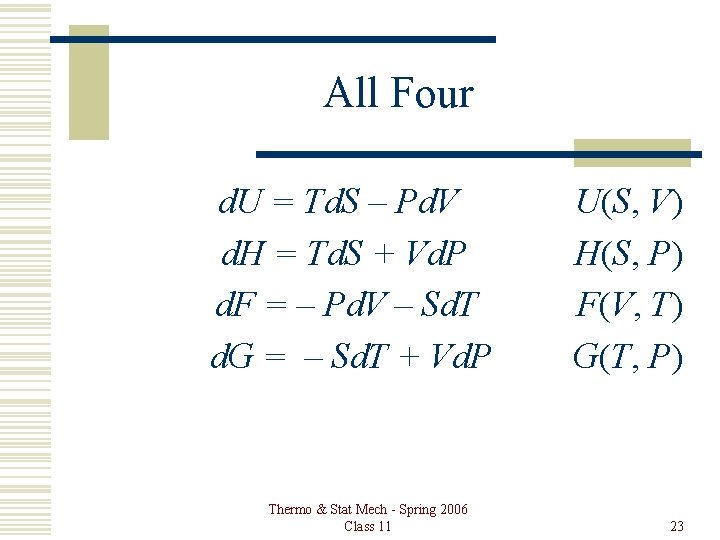
All Four d. U = Td. S – Pd. V d. H = Td. S + Vd. P d. F = – Pd. V – Sd. T d. G = – Sd. T + Vd. P Thermo & Stat Mech - Spring 2006 Class 11 U(S, V) H(S, P) F(V, T) G(T, P) 23

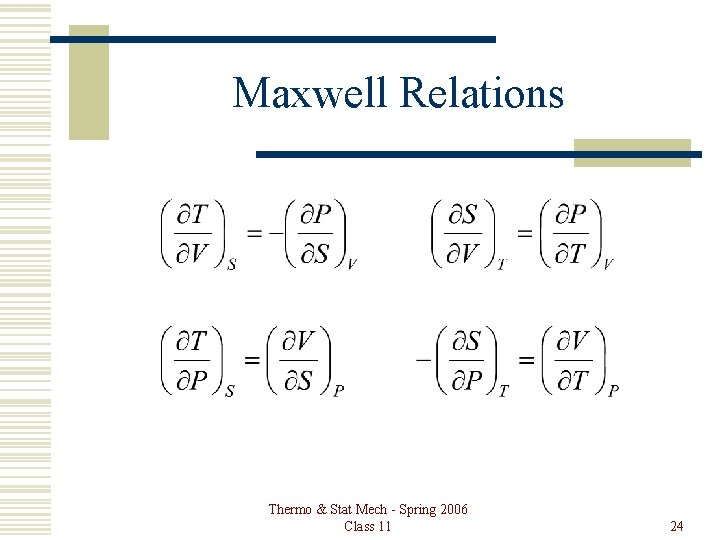
Maxwell Relations Thermo & Stat Mech - Spring 2006 Class 11 24

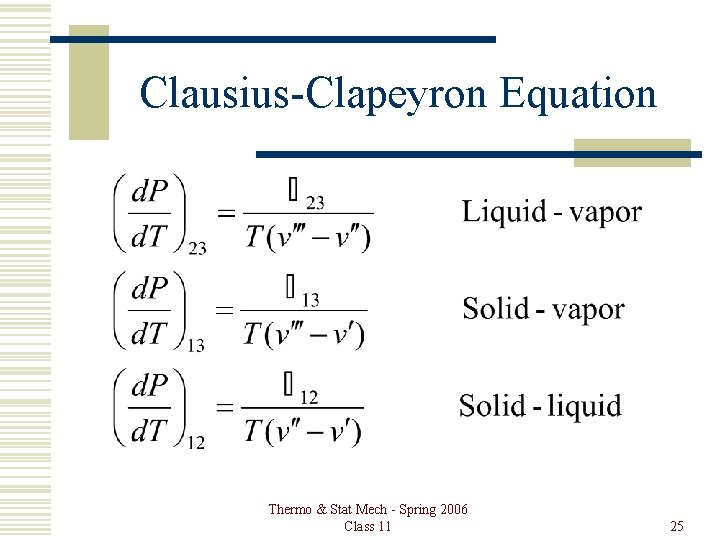
Clausius-Clapeyron Equation Thermo & Stat Mech - Spring 2006 Class 11 25
 Thermodynamics and statistical mechanics
Thermodynamics and statistical mechanics Thermodynamics and statistical mechanics
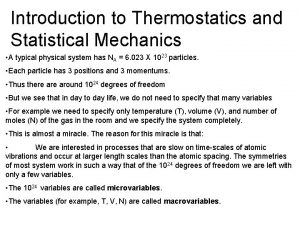
Thermodynamics and statistical mechanics Statistical thermodynamics is a study of
Statistical thermodynamics is a study of Statistical thermodynamics
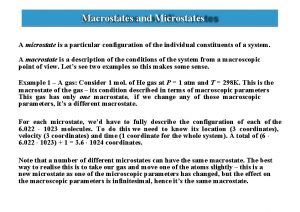
Statistical thermodynamics Macrostate and microstate in statistical mechanics
Macrostate and microstate in statistical mechanics Partition function in statistical mechanics
Partition function in statistical mechanics Statistical mechanics
Statistical mechanics Classical equipartition
Classical equipartition Partition function in statistical mechanics
Partition function in statistical mechanics Gibbs free energy
Gibbs free energy Partition function in statistical mechanics
Partition function in statistical mechanics Introduction to quantum statistical mechanics
Introduction to quantum statistical mechanics Statistical mechanics of deep learning
Statistical mechanics of deep learning Statistical mechanics
Statistical mechanics Lesson 10 thermodynamics unit review
Lesson 10 thermodynamics unit review Statistical physics quiz
Statistical physics quiz Bp statistical review of world energy 2009
Bp statistical review of world energy 2009 Bp statistical review
Bp statistical review Statistical pattern recognition a review
Statistical pattern recognition a review Bp statistical review of world energy 2014
Bp statistical review of world energy 2014 Body mechanics quiz
Body mechanics quiz Normalize wave function e^ix
Normalize wave function e^ix Kontinuitetshantering
Kontinuitetshantering Novell typiska drag
Novell typiska drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för