Thermodynamics and Metabolism Metabolism q Metabolism Catabolism Anabolism

Thermodynamics and Metabolism

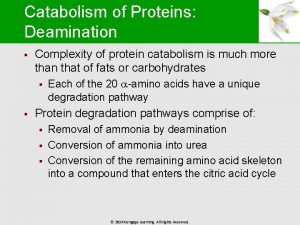
Metabolism q Metabolism = Catabolism + Anabolism q Catabolic reactions are energy yielding • breakdown of more-complex molecules into simpler ones q Anabolic reactions are energy requiring • building up of simpler molecules into morecomplex ones
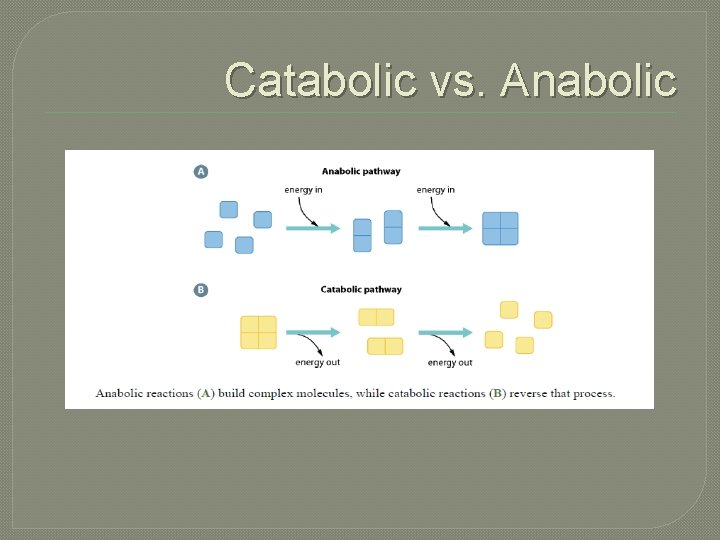
Catabolic vs. Anabolic
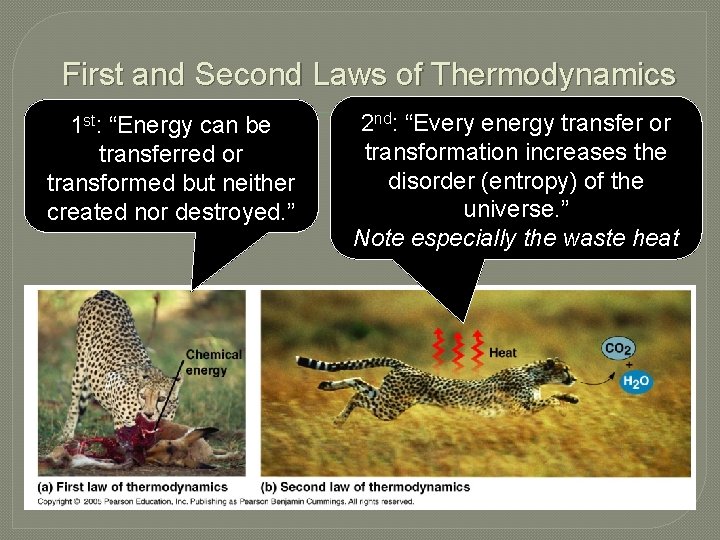
First and Second Laws of Thermodynamics 1 st: “Energy can be transferred or transformed but neither created nor destroyed. ” 2 nd: “Every energy transfer or transformation increases the disorder (entropy) of the universe. ” Note especially the waste heat

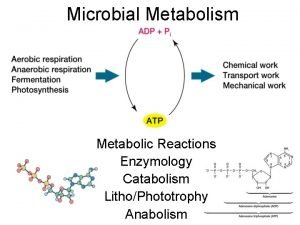
Energy in the Biosphere q Organisms take in energy & transduce it to new forms (1 st law) q As energy transducers, organisms are less than 100% efficient (2 nd law)

Organisms employ this energy to: • • • Grow Protect Themselves Repair Themselves Compete with other Organisms Make new Organisms (i. e. , babies)

�In the process, organisms generate waste chemicals & heat �Organisms create local regions of order at the expense of the total energy found in the Universe!!! We are Energy Parasites!

Kinetic and Potential Energy

q First Law of Thermodynamics: • Energy can be neither created nor destroyed • Therefore, energy “generated” in any system is energy that has been transformed from one state to another (e. g. , chemically stored energy transformed to heat)

� Second Law of Thermodynamics: • Efficiencies of energy transformation never equal 100% • Conversion to heat is the ultimate fate of chemical energy
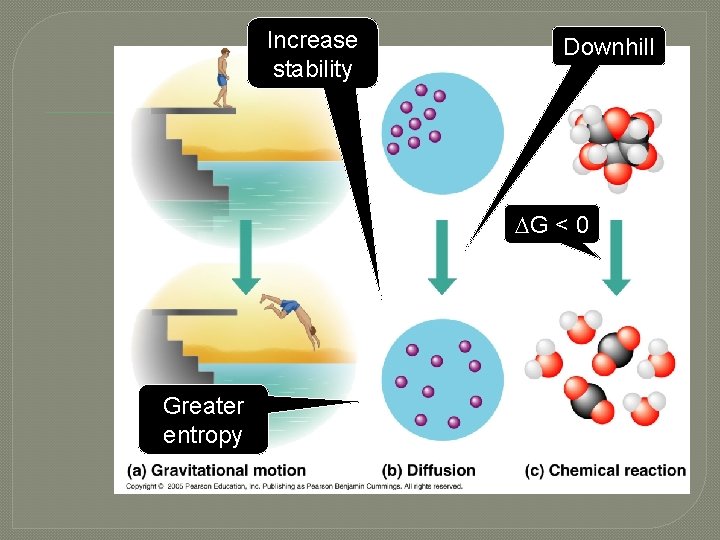
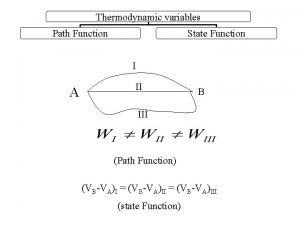
Increase stability Downhill G < 0 Greater entropy

“Food” Potential energy Forward reaction Spontaneous Waste heat Work

Types of Reactions
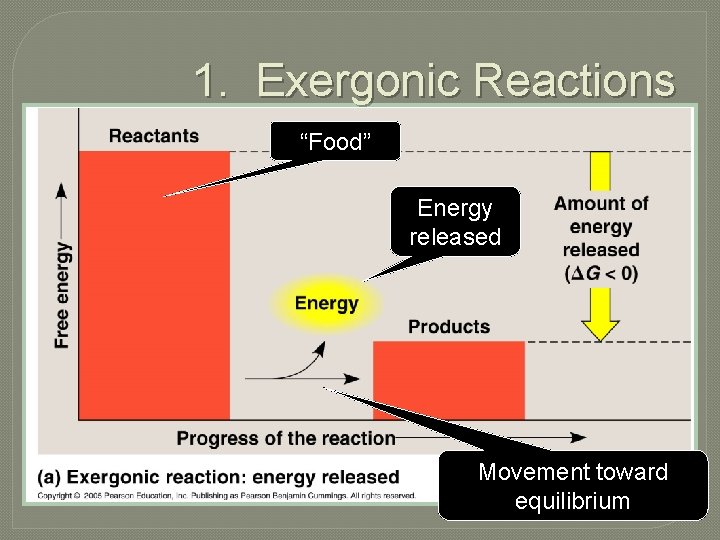
1. Exergonic Reactions “Food” Energy released Movement toward equilibrium

• Decrease in Gibbs free energy (- G) • Increase in stability • Spontaneous (gives off net energy upon going forward) • Downhill (toward center of gravity well, e. g. , of Earth) • Movement towards equilibrium • Coupled to ATP production (ADP phosphorylation) • Catabolism
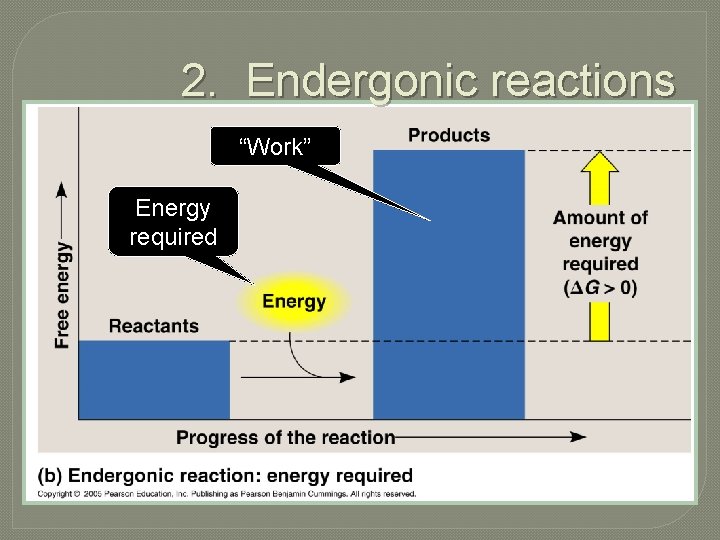
2. Endergonic reactions “Work” Energy required


Coupling Reactions Exergonic reactions can supply energy for endergonic reactions
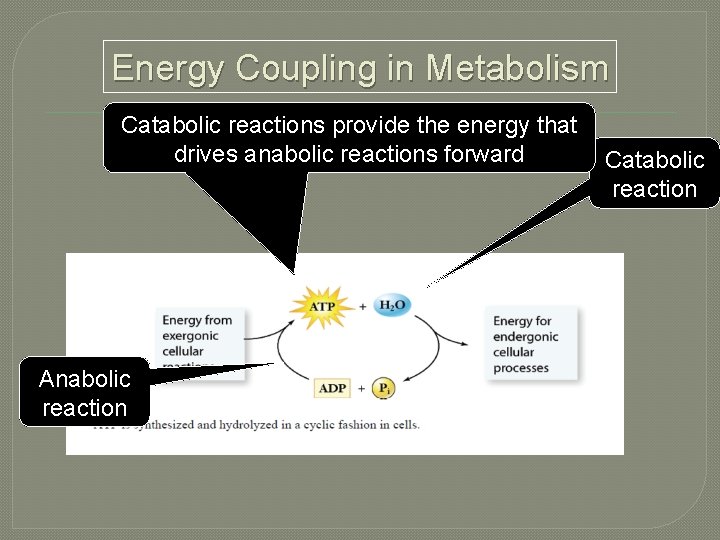
Energy Coupling in Metabolism Catabolic reactions provide the energy that drives anabolic reactions forward Anabolic reaction Catabolic reaction
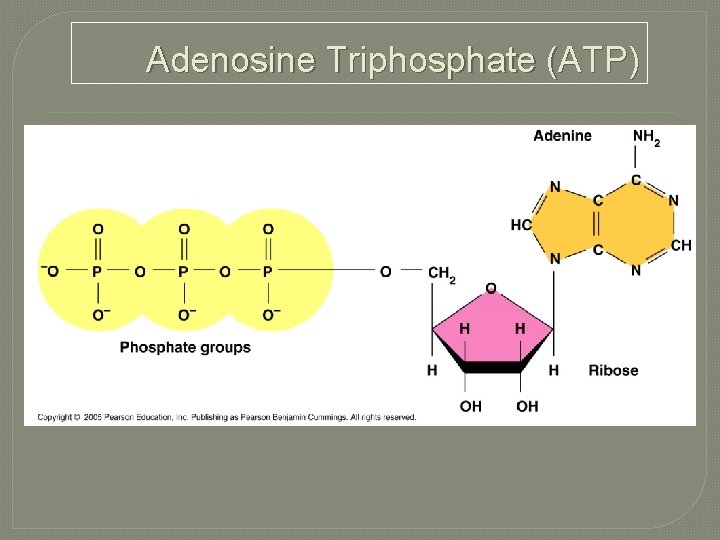
Adenosine Triphosphate (ATP)
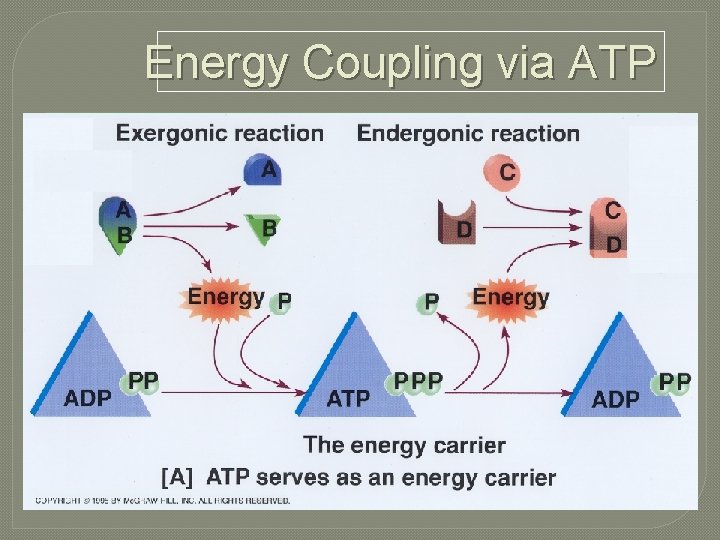
Energy Coupling via ATP
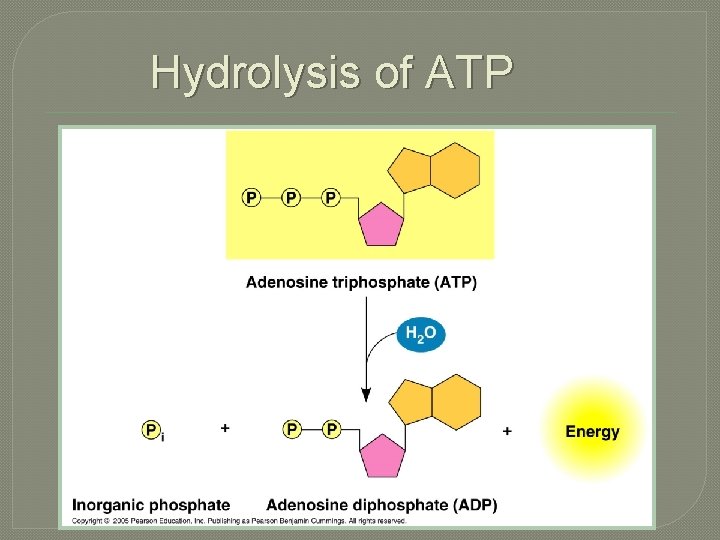
Hydrolysis of ATP
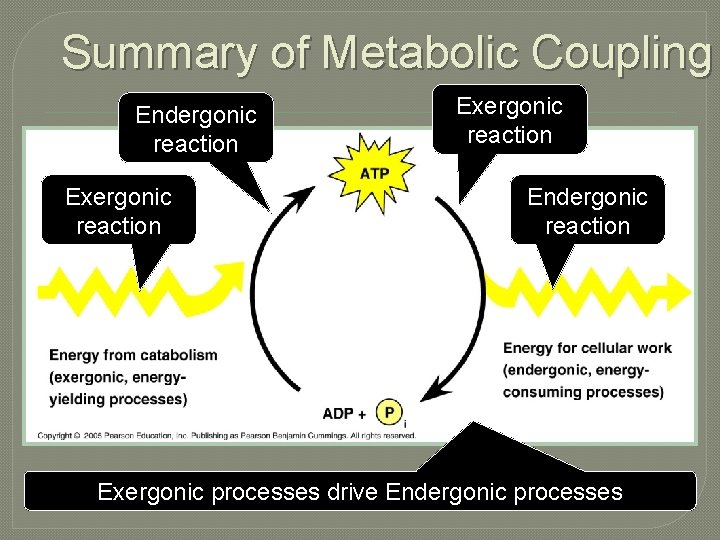
Summary of Metabolic Coupling Endergonic reaction Exergonic reaction Endergonic reaction Exergonic processes drive Endergonic processes
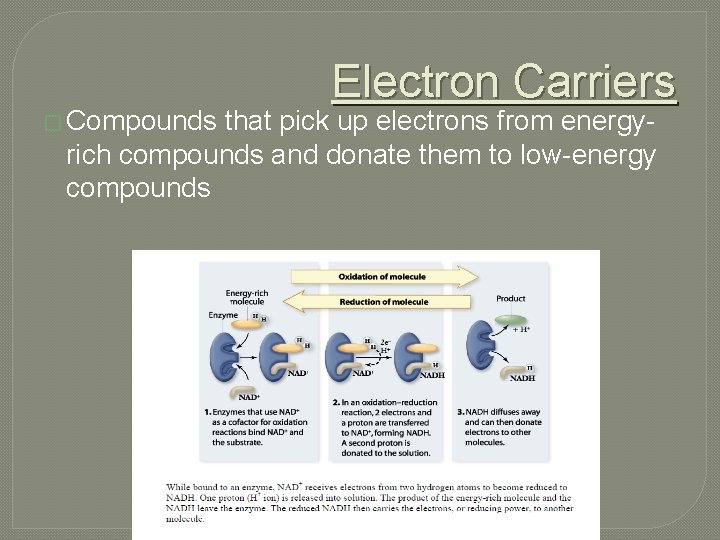
� Compounds Electron Carriers that pick up electrons from energyrich compounds and donate them to low-energy compounds

�Electrons that pass from one to another carry energy with them, so the reduced form of a molecule is always at a higher energy level than the oxidized form
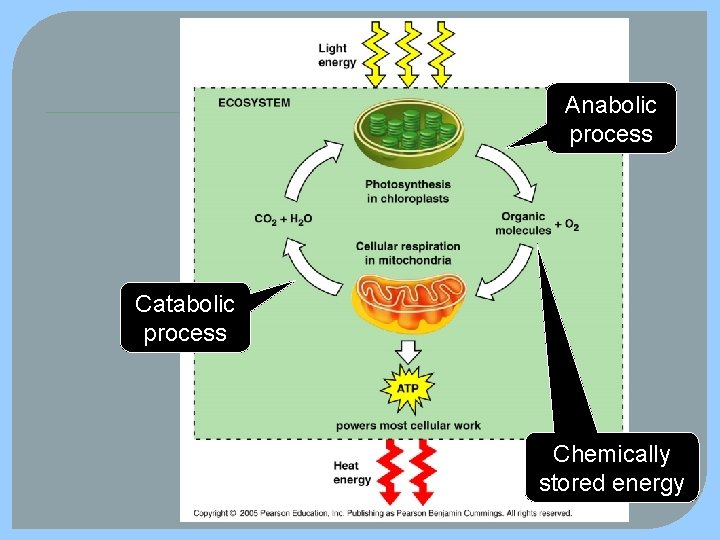
Anabolic process Catabolic process Chemically stored energy
- Slides: 26