Thermodynamics and Kinetics study of growth behavior of

Thermodynamics and Kinetics study of growth behavior of sono-electrodeposited Cu thin films Sabita Rout, A. Mallik, B. C. Ray Sabita. swain 1@gmail. com archananitrkl@gmail. com drbcray@gmail. com , Department of Metallurgical and Materials Engineering National Institute of Technology, Rourkela

SEQUENCE OF PRESENTATION § Growth of thin films – an insight § The growth parable § Sono-electrodeposition technique § Experimental /Results and discussion § Conclusions § References
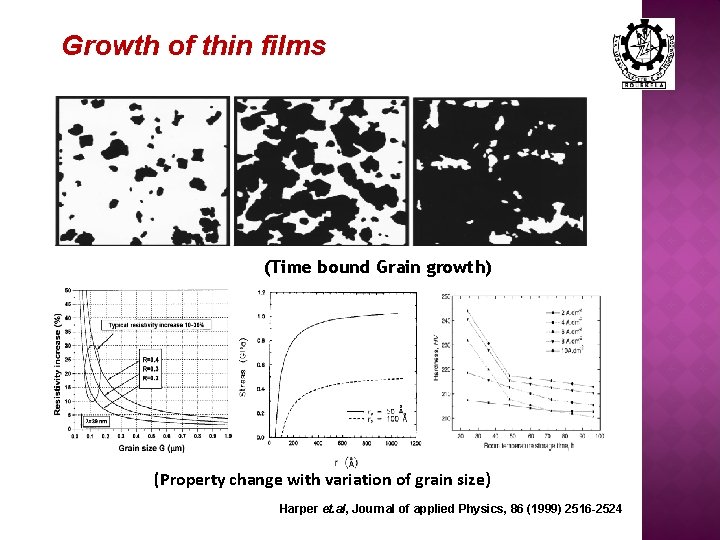
Growth of thin films (Time bound Grain growth) (Property change with variation of grain size) Harper et. al, Journal of applied Physics, 86 (1999) 2516 -2524
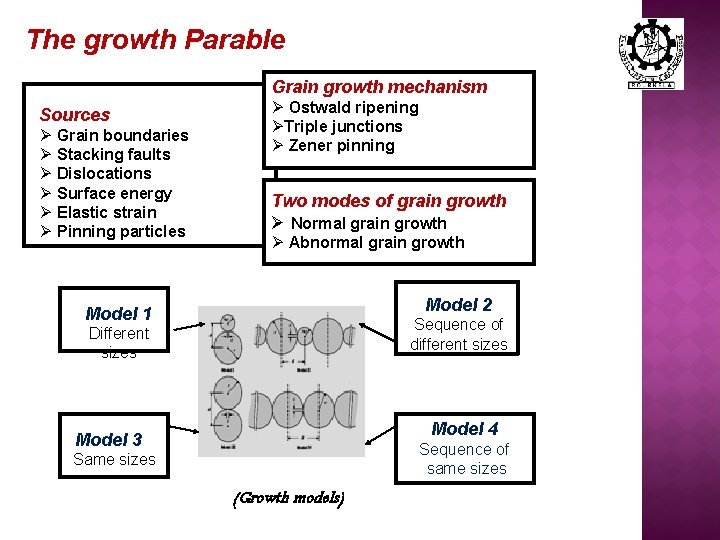
The growth Parable Grain growth mechanism Sources Ø Grain boundaries Ø Stacking faults Ø Dislocations Ø Surface energy Ø Elastic strain Ø Pinning particles Ø Ostwald ripening ØTriple junctions Ø Zener pinning Two modes of grain growth Ø Normal grain growth Ø Abnormal grain growth Model 2 Model 1 Sequence of different sizes Different sizes Model 4 Model 3 Sequence of same sizes Same sizes (Growth models)
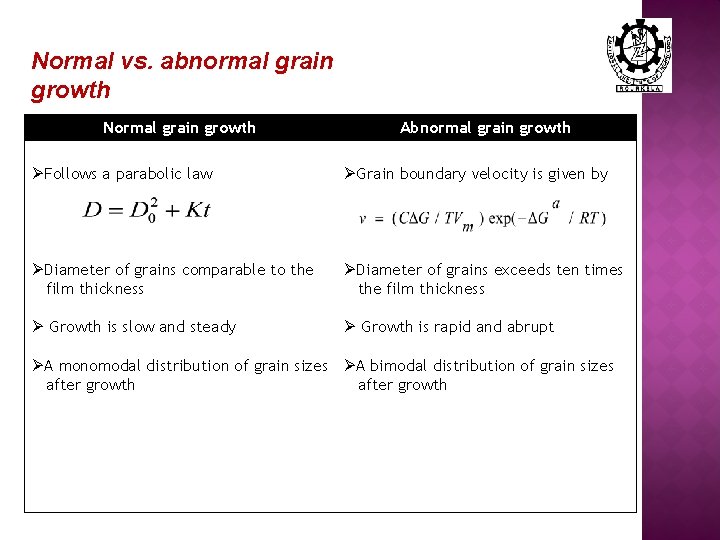
Normal vs. abnormal grain growth Normal grain growth Abnormal grain growth ØFollows a parabolic law ØGrain boundary velocity is given by ØDiameter of grains comparable to the film thickness ØDiameter of grains exceeds ten times the film thickness Ø Growth is slow and steady Ø Growth is rapid and abrupt ØA monomodal distribution of grain sizes ØA bimodal distribution of grain sizes after growth
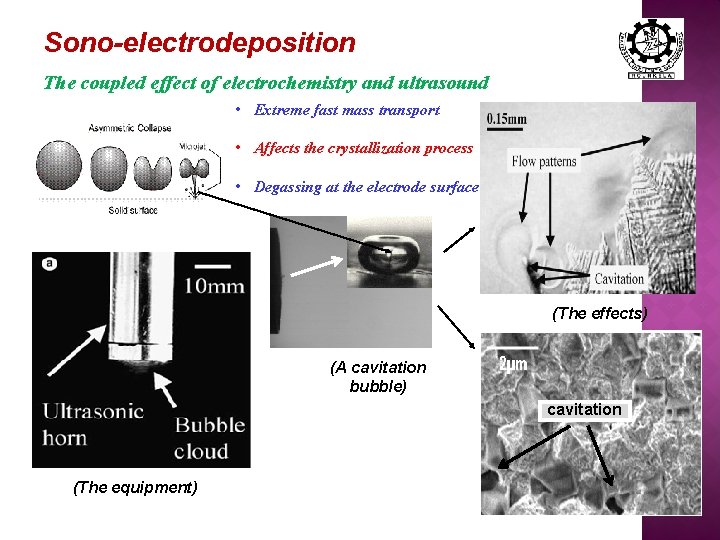
Sono-electrodeposition The coupled effect of electrochemistry and ultrasound • Extreme fast mass transport • Affects the crystallization process • Degassing at the electrode surface (The effects) (A cavitation bubble) cavitation (The equipment)
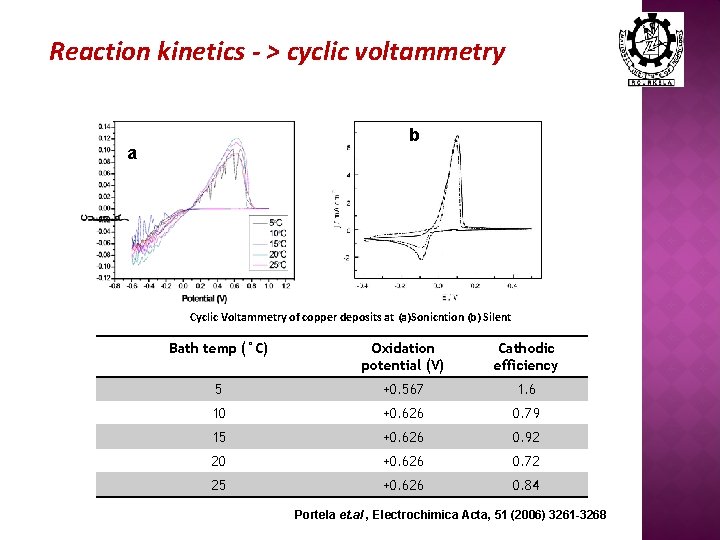
Reaction kinetics - > cyclic voltammetry b a Cyclic Voltammetry of copper deposits at (a)Sonicntion (b) Silent Bath temp (°C) Oxidation potential (V) Cathodic efficiency 5 +0. 567 1. 6 10 +0. 626 0. 79 15 +0. 626 0. 92 20 +0. 626 0. 72 25 +0. 626 0. 84 Portela et. al , Electrochimica Acta, 51 (2006) 3261 -3268
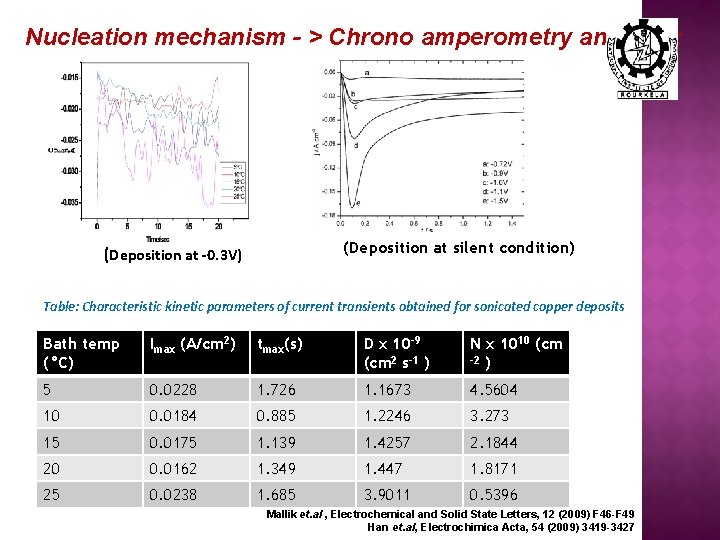
Nucleation mechanism - > Chrono amperometry analysis: (Deposition at silent condition) (Deposition at -0. 3 V) Table: Characteristic kinetic parameters of current transients obtained for sonicated copper deposits 2 Bath temp 20 °C Imax (A/cm ) (°C) tmax(s) D x 10 -9 (cm 2 s-1 ) N x 1010 (cm -2 ) 5 0. 0228 1. 726 1. 1673 4. 5604 10 0. 0184 0. 885 1. 2246 3. 273 15 0. 0175 1. 139 1. 4257 2. 1844 20 0. 0162 1. 349 1. 447 1. 8171 25 0. 0238 1. 685 3. 9011 0. 5396 Mallik et. al , Electrochemical and Solid State Letters, 12 (2009) F 46 -F 49 Han et. al, Electrochimica Acta, 54 (2009) 3419 -3427
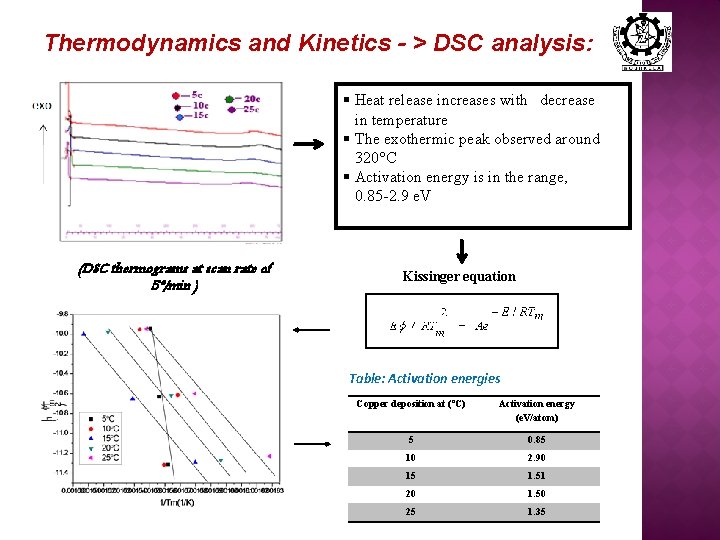
Thermodynamics and Kinetics - > DSC analysis: § Heat release increases with decrease in temperature § The exothermic peak observed around 320°C § Activation energy is in the range, 0. 85 -2. 9 e. V (DSC thermograms at scan rate of 5°/min ) Kissinger equation Table: Activation energies Copper deposition at (°C) Activation energy (e. V/atom) 5 0. 85 10 2. 90 15 1. 51 20 1. 50 25 1. 35
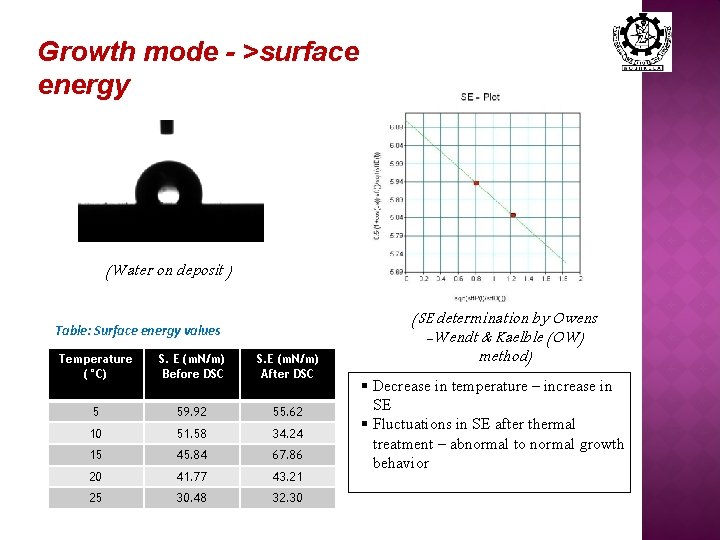
Growth mode - >surface energy (Water on deposit ) Table: Surface energy values Temperature (°C) S. E (m. N/m) Before DSC S. E (m. N/m) After DSC 5 59. 92 55. 62 10 51. 58 34. 24 15 45. 84 67. 86 20 41. 77 43. 21 25 30. 48 32. 30 (SE determination by Owens -Wendt & Kaelble (OW) method) § Decrease in temperature – increase in SE § Fluctuations in SE after thermal treatment – abnormal to normal growth behavior
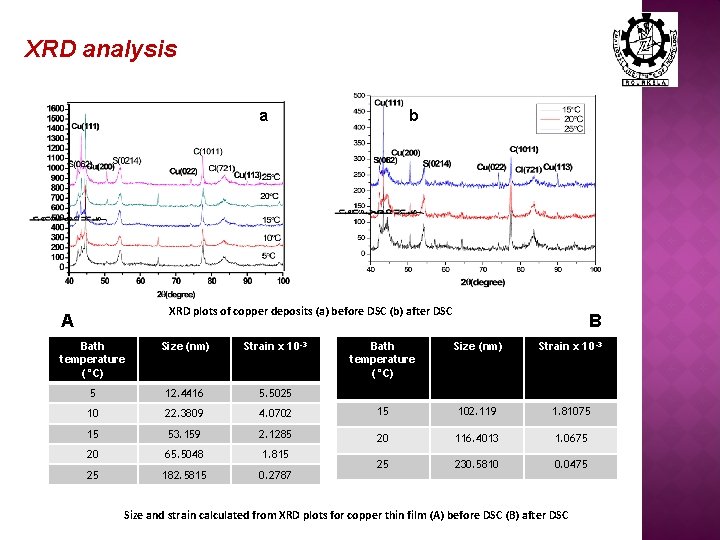
XRD analysis a b XRD plots of copper deposits (a) before DSC (b) after DSC A Bath temperature (°C) Size (nm) Strain x 10 -3 5 12. 4416 5. 5025 10 22. 3809 15 B Bath temperature (°C) Size (nm) Strain x 10 -3 4. 0702 15 102. 119 1. 81075 53. 159 2. 1285 20 116. 4013 1. 0675 20 65. 5048 1. 815 25 182. 5815 0. 2787 25 230. 5810 0. 0475 Size and strain calculated from XRD plots for copper thin film (A) before DSC (B) after DSC
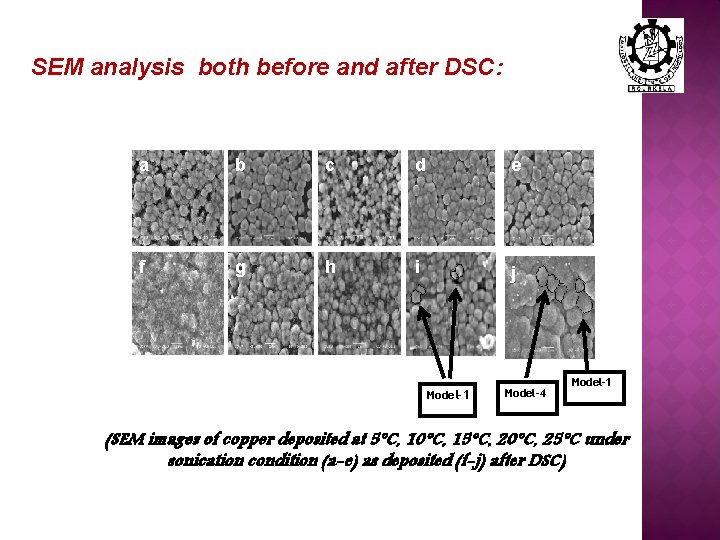
SEM analysis both before and after DSC: a a b c d e f g h i jj c d Model-1 Model-4 Model-1 (SEM images of copper deposited at 5°C, 10°C, 15°C, 20°C, 25°C under sonication condition (a-e) as deposited (f-j) after DSC)

Conclusions Ø Better adherence of deposit by sono-electrodeposition. Ø The appearance of exothermic peak signifies occurrence of grain growth. Ø Determination of activation energy provides information about the kinetics of grain growth. Ø Whether proposed growth mechanism are the correct way to explore grain growth, will remain unclear until further investigations down to single grain or monolayer films

References 1. J. M. Zhang, K. W. Xu, V. Ji. Competition between surface and strain energy during grain growth in free-standing and attached Ag and Cu films on Si substrates. Applied surface science 187 (2002) 60 -67. 2. J. M. E. Harper, C. Cabral, P. C. Andricacos, L. Gignac, I. C. Noyan. Mechanisms for microstructure evolution in electroplated copper thin films near room temperature. Journal of applied physics 86 (1999) 2516 -2525. 3. F. P. Luce, P. Fichtner, L. F. Schelp. Abnormal grain growth behavior in nanostructured Al thin films on Si. O 2/Si substrate. Material Research Society 1150 (2009) RR 03 -06. 4. C. Detavernier, S. Rossnagel, C. Noyan, S. Cabral. Thermodynamics and kinetics of room-temperature microstructural evolution in copper films. Journal of applied physics 94 (2003) 2874 -2881. 5. A. Mallik, A. Bankoti, B. C. Ray. A Study on the Modification of Conventional Electrochemical Crystallization under Sonication: The Phenomena of Secondary Nucleation. Electrochemical and Solid-State Letters 12 (2009) F-46 -F-49. 6. R. Cow, R. Blindt, R. Chivers, M. Povey. A study on the primary and secondary nucleation of ice by power ultrasound. Ultrasonics 43 (2005) 227 -230. 7. D. Bera, S. C. Kuiry, S. Seal. Kinetics and Growth Mechanism of Electrodeposited Palladium Nanocrystallites. Journal of Physical Chemistry 108 (2004) 556 -562. 8. R. Finsy. On the Critical Radius in Ostwald Ripening. Langmuir 20 (2004) 2975 -2976. 9. K. B. Yin, Y. D. Xia, C. Y. Chan, W. Q. Zhang. The kinetics and mechanism of room-temperature microstructural evolution in electroplated copper foils. Scripta Materialia 58 (2008) 65 -68. 10. S. Villain, P. Knauth, G. Schwitzgebel. Electrodeposition of Nanocrystalline Silver: Study of Grain Growth by Measurement of Reversible Electromotive Force. Journal of Physical Chemistry 101 (1997) 7452 -7454. 11. H. E. Kissinger. Reaction Kinetics in Differential Thermal Analysis. Analytical Chemistry 29 (1957) 1702 -1706. 12. L. Zhou, H. Zhang, D. J. Srolovitz. A size effect in grain boundary migration: A molecular dynamics study of bicrystal thin films. Acta Materialia 53 (2005) 5273 -5279. 13. S. K. Donthu, M. Vora, S. K. Lahiri, C. V. Thompson. Activation Energy Determination for Recrystallization in Electroplated-copper films using Differential Scanning Calorimetry. Journal of Electronic Materials 32 (2003) 531 -534. 14. P. Knauth, A. Charai, P. Gas. Grain growth of pure nickel and of Ni-Si solid solution studied by Differential Scanning Calorimetry of nanometer-sized crystals. Scripta Metallurgica Materialia 28 (1993) 325 -330.

- Slides: 15