THERMODYNAMICS 1 CHEMICAL ENGINEERING THERMODYNAMICS THEORY Thermodynamics born

THERMODYNAMICS 1 CHEMICAL ENGINEERING THERMODYNAMICS
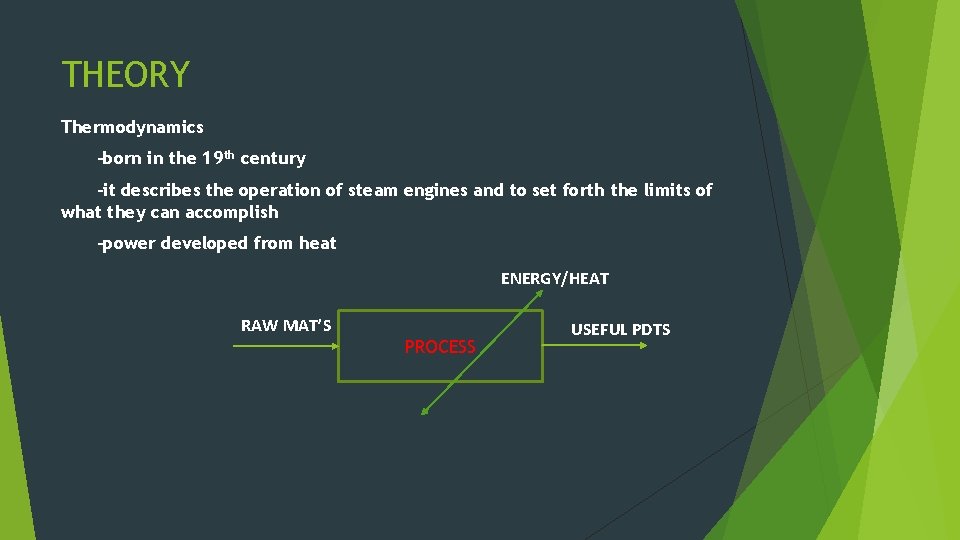
THEORY Thermodynamics -born in the 19 th century -it describes the operation of steam engines and to set forth the limits of what they can accomplish -power developed from heat ENERGY/HEAT RAW MAT’S PROCESS USEFUL PDTS

THEORY Chemical Engineering Thermodynamics deals with the “microthermo” Thermo-“heat” Dynamics-“motion” Internal energy (U) -refers to the energy of the molecules internal to the substance -does not include energy that it may possess as a result of its macroscopic position or movement Internal kinetic energy-atom’s mobility Internal potential energy-atom is at rest; molecules are at rest

THEORY Open System-matter and energy can flow into and out of the system boundary Closed system-the boundary of the system does not permit the transfer of matter between the system and its surroundings Isolated system-nothing can flow into and out of the system boundary Path functions-functions dependent on the path State functions-not dependent on the path

THEORY Work (W) -is performed whenever a force acts through a distance Reversible Process -process is reversible when its direction can be reversed at any point by an infinitesimal change in external conditions Second Law of Newton: α = gc (F/m) F=ma/gc Energy Types of energy: Potential Kinetic
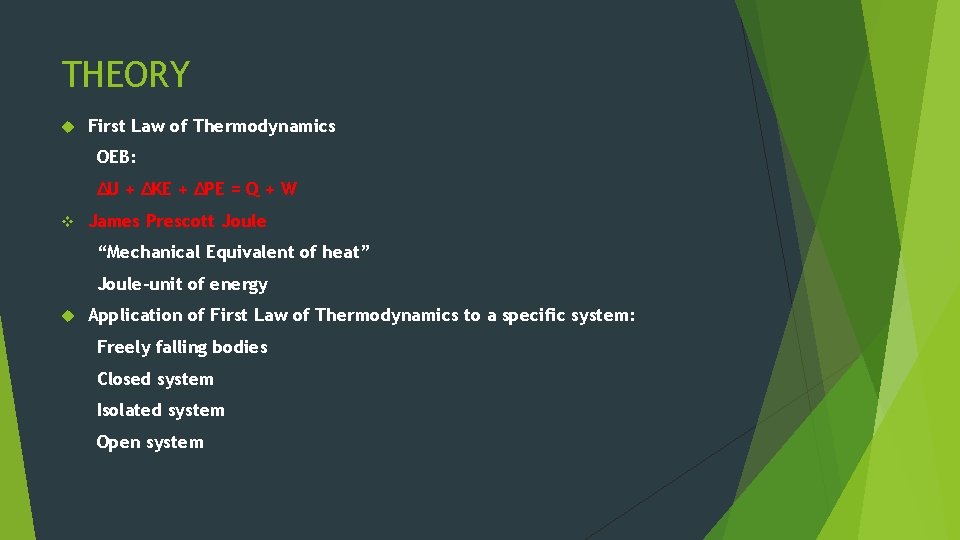
THEORY First Law of Thermodynamics OEB: ΔU + ΔKE + ΔPE = Q + W v James Prescott Joule “Mechanical Equivalent of heat” Joule-unit of energy Application of First Law of Thermodynamics to a specific system: Freely falling bodies Closed system Isolated system Open system

EQUATIONS Internal Energy: U=Ukinetic + Upotential Work: W=-Popposing(V 2 – V 1) d. W=-Popposingd. V General Equations: ΔU=n. Cv ΔT ΔH=n. Cp ΔT Second law of motion: F=ma/gc Energy: W= ΔKE W= ΔPE First Law of Thermodynamics; OEB: ΔU + ΔKE + ΔPE = Q + W

SAMPLE PROBLEM #1 Steam enters a pipe at 400˚C with a pressure of 4. 0 MPa. It is then discharged with a temperature of 392˚C and a pressure of 3. 5 MPa. The amount of heat liberated by the system is 8. 5 k. J/kg. a. b. What kind of system? mass flowrate of the steam if the pipe’s diameter 20 cm
- Slides: 8