Thermodynamics 1 2 2 Heat of Formation 2





- Slides: 10

Thermodynamics 1. 2. 2 Heat of Formation

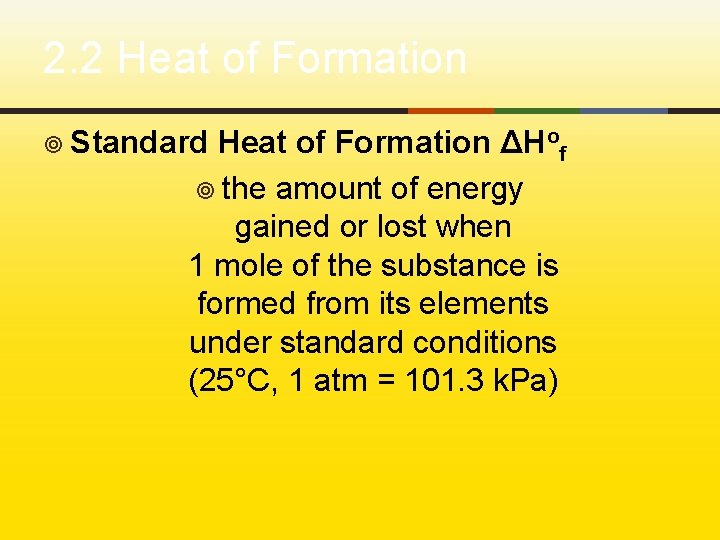
2. 2 Heat of Formation ¥ Standard Heat of Formation ΔHof ¥ the amount of energy gained or lost when 1 mole of the substance is formed from its elements under standard conditions (25°C, 1 atm = 101. 3 k. Pa)

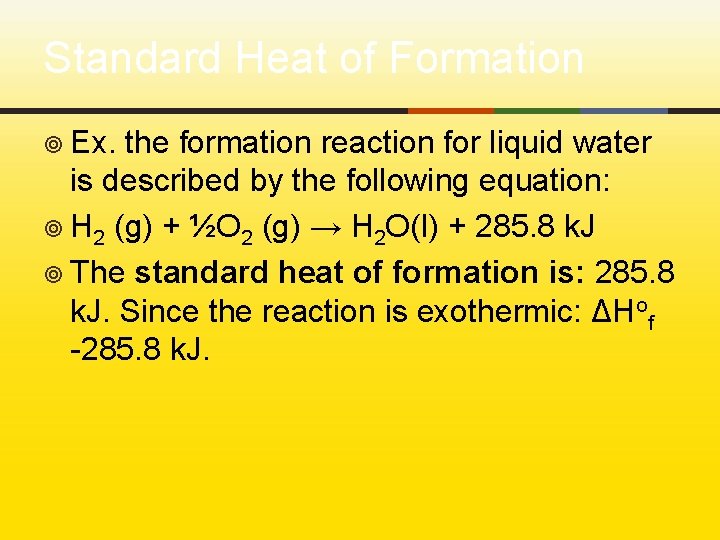
Standard Heat of Formation ¥ Ex. the formation reaction for liquid water is described by the following equation: ¥ H 2 (g) + ½O 2 (g) → H 2 O(l) + 285. 8 k. J ¥ The standard heat of formation is: 285. 8 k. J. Since the reaction is exothermic: ΔHof -285. 8 k. J.

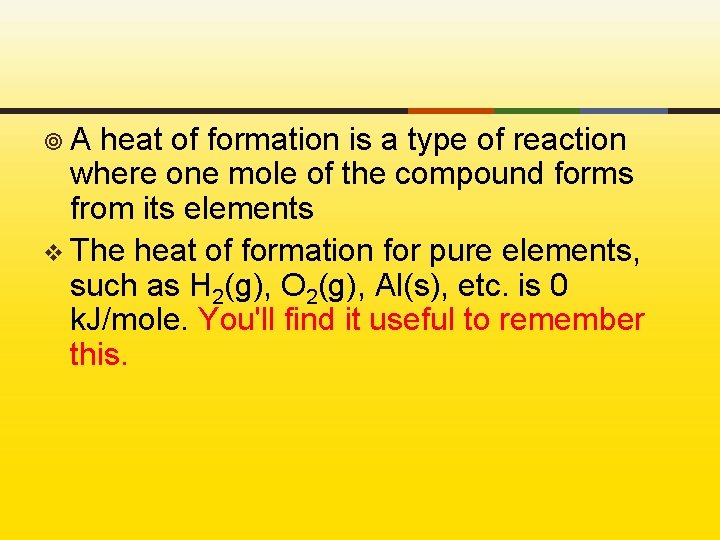
¥A heat of formation is a type of reaction where one mole of the compound forms from its elements v The heat of formation for pure elements, such as H 2(g), O 2(g), Al(s), etc. is 0 k. J/mole. You'll find it useful to remember this.

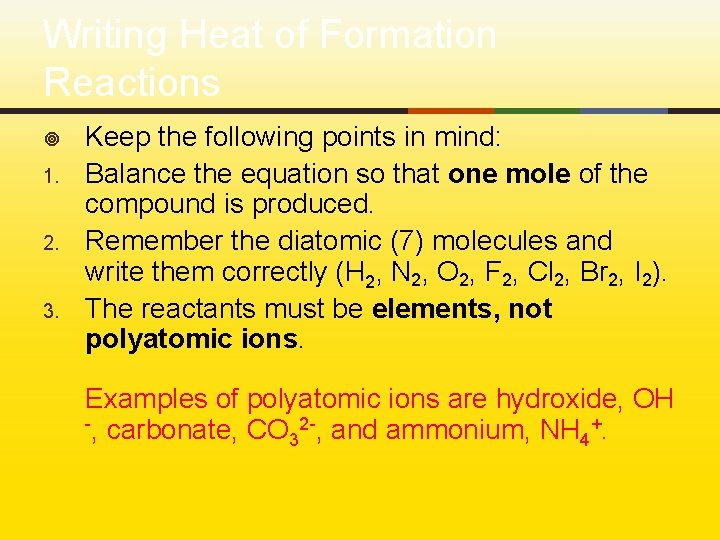
Writing Heat of Formation Reactions ¥ 1. 2. 3. Keep the following points in mind: Balance the equation so that one mole of the compound is produced. Remember the diatomic (7) molecules and write them correctly (H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2). The reactants must be elements, not polyatomic ions. Examples of polyatomic ions are hydroxide, OH -, carbonate, CO 2 -, and ammonium, NH +. 3 4

Review ¥ ¥ H 2(g) + ½O 2(g) → H 2 O(l) + 285 k. J. If 285. 8 k. J of energy are released during the formation of one mole of H 2 O(l), how much energy do you imagine would be released if two moles of water were produced?

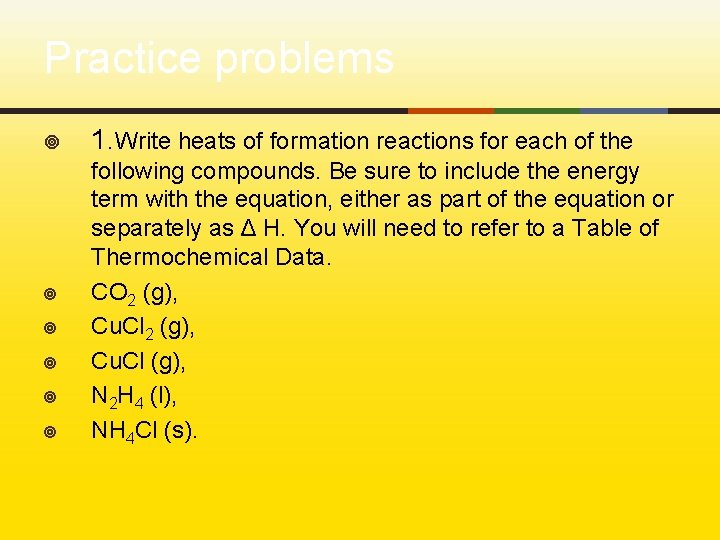
Practice problems ¥ 1. Write heats of formation reactions for each of the ¥ following compounds. Be sure to include the energy term with the equation, either as part of the equation or separately as Δ H. You will need to refer to a Table of Thermochemical Data. CO 2 (g), Cu. Cl (g), N 2 H 4 (l), NH 4 Cl (s). ¥ ¥

Example The standard heat of formation, ΔHof, for sulfur dioxide (SO 2) is -297 k. J/mol. How many k. J of energy are given off when 25. 0 g of SO 2 (g) is produced from its elements?

Example The heat of reaction for the combustion of 1 mol of ethyl alcohol is -9. 50 × 102 k. J: C 2 H 5 OH (l) + 3 O 2 (g) → 2 CO 2 (g) + 3 H 2 O (l) + 9. 5 × 102 k. J How much heat is produced when 11. 5 g of alcohol is burned?

Example ΔH for the complete combustion of 1 mol of propane is -2. 22 × 103 k. J: C 3 H 8 (g) + 5 O 2 (g) → 3 CO 2 (g) + 4 H 2 O (l) Calculate the heat of reaction for the combustion of 33. 0 g of propane.