Thermodynamic Processes ENTROPY GIBBS FREE ENERGY Entropy S

Thermodynamic Processes Ø ENTROPY Ø GIBBS FREE ENERGY

Entropy, S A measure of disorder

The Laws of Thermodynamics Zeroeth If two systems are in thermal equilibrium with a third system, they are in thermal equilibrium with each other.

The Laws of Thermodynamics First Law of Conservation of Energy In an isolated system, energy cannot be created or destroyed.

The Laws of Thermodynamics Second In an isolated system, entropy always increases. ***THIS IS OUR FOCUS TODAY!!!

The Laws of Thermodynamics Third As the temperature approaches absolute zero, the entropy of a system approaches a constant value.
Entropy In general the universe tends to move toward release of energy and greater entropy. 7

Entropy The statistical interpretation of thermodynamics was pioneered by James Clerk Maxwell (1831– 1879) …and brought to fruition by the Austrian physicist Ludwig Boltzmann(1844– 1906). 8

Check out their personal entropy change over time! 9

Entropy Spontaneous chemical processes often result in a final state that is more Disordered or Random than the original. Reaction of potassium metal with water. The products are more randomly distributed than the reactants. Spontaneity = fn(randomness) 10
The 2 nd Law of Thermo In an isolated system, entropy always increases. What are the odds of a Royal Flush? ! True because there exist more possibilities for disorder than for order.
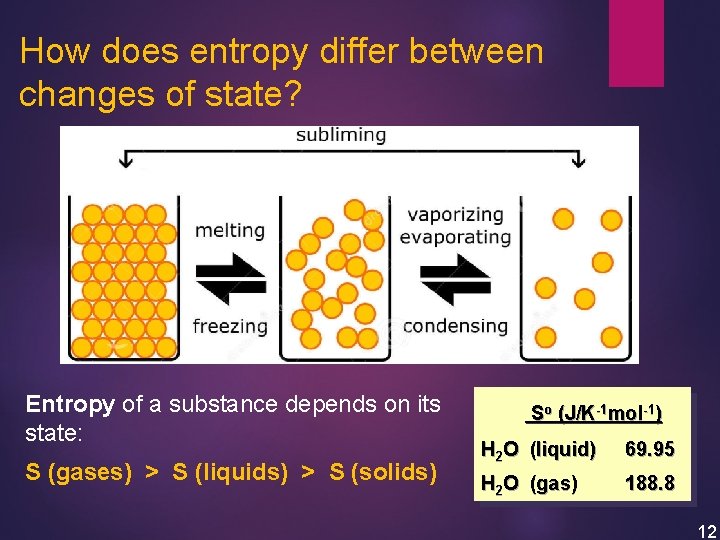
How does entropy differ between changes of state? Entropy of a substance depends on its state: S (gases) > S (liquids) > S (solids) So (J/K-1 mol-1) H 2 O (liquid) 69. 95 H 2 O (gas) 188. 8 12
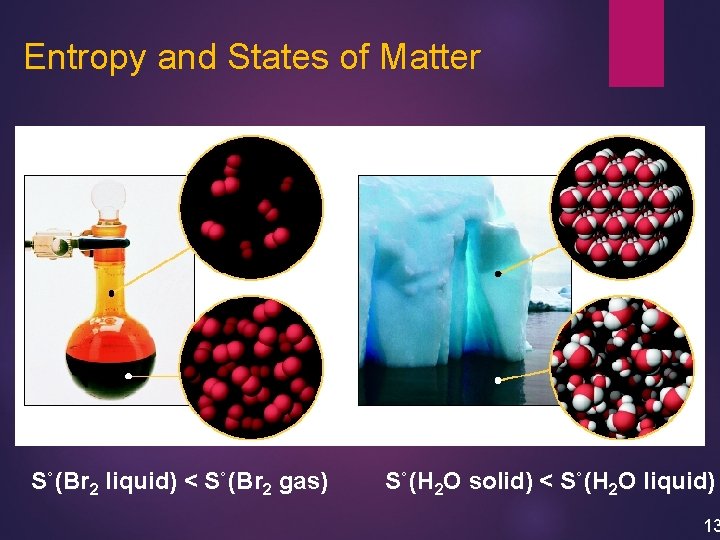
Entropy and States of Matter S˚(Br 2 liquid) < S˚(Br 2 gas) S˚(H 2 O solid) < S˚(H 2 O liquid) 13
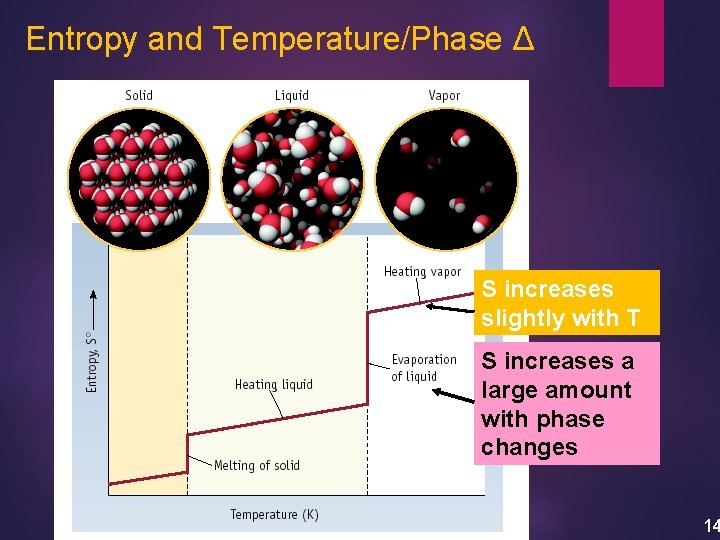
Entropy and Temperature/Phase Δ S increases slightly with T S increases a large amount with phase changes 14
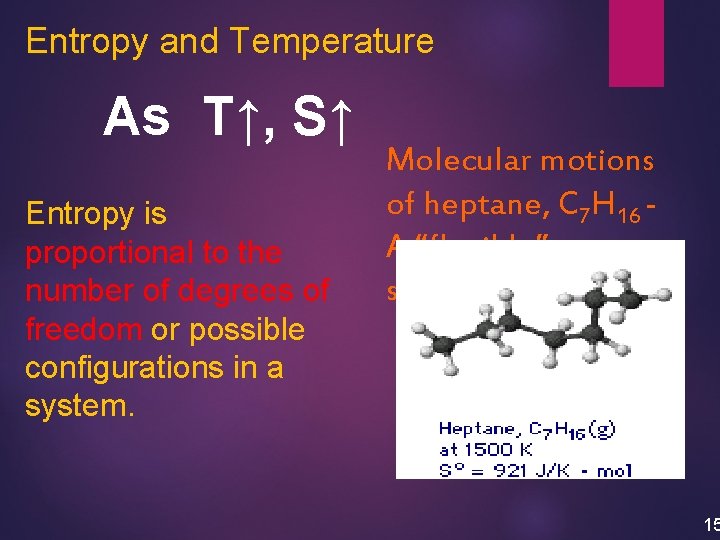
Entropy and Temperature As T↑, S↑ Entropy is proportional to the number of degrees of freedom or possible configurations in a system. Molecular motions of heptane, C 7 H 16 A “flexible” structure 15

The symbol for the change in disorder or entropy is given by the symbol, DS. AS T↑… S↑ OR DS>0

Entropy is Disorder Which of the following represent DS>0? Ø Dissolving salt in water. Mixing different types of particles. Ø Evaporation of water. A change in state where the distance between particles increases. Ø Increase in temperature. Increased movement of particles. Ø 2 KCl. O 3 2 KCl + 3 O 2 Incr # of particles. 17

Some generalizations S↑ as substance changes from solid to liquid to gas and vice versa. S↑ as a pure solid or liquid dissolves in a solvent. S↓ with increasing molecular complexity.

Entropy States The greatest increase in entropy is usually found when there is an increase of particles in the gaseous state. The more disordered a system becomes the more positive the value for DS will be. Systems that become more ordered have negative DS values. 19

Standard Entropy Values standard entropy change, DSo, of a substance is the entropy change per mole that occurs when heating a substance from 0 K to the standard temperature of 298 K. The Unlike enthalpy, absolute entropy changes can be measured. 20
Standard Entropy Values Like enthalpy, entropy is a state function. The amount of entropy in a pure substance is a function of T, P, and # molecules (n). The change in entropy is the difference between the products and the reactants. o DS =S o S products- S o S reactants 21
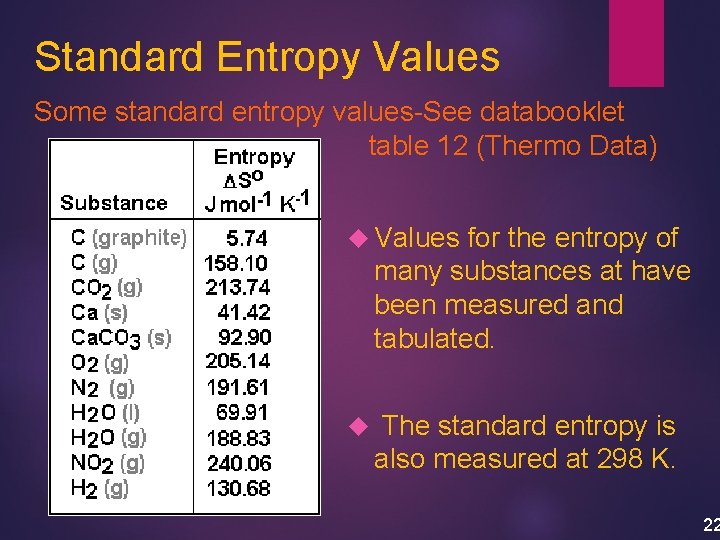
Standard Entropy Values Some standard entropy values-See databooklet table 12 (Thermo Data) Values for the entropy of many substances at have been measured and tabulated. The standard entropy is also measured at 298 K. 22

…and now for a little practice!

Gibbs Free Energy, G A measure of the energy associated w/ rxn available to do work 24

Spontaneity A chemical reaction is spontaneous if… It occurs without any additional work being done on the system ü it results in the system moving from a less stable to a more stable state ü Thermodynamically favorable reaction ü 2 Energy Factors: Spontaneity is a function of both an enthalpy factor and an entropy factor and can be expressed as Gibbs Free Energy 25
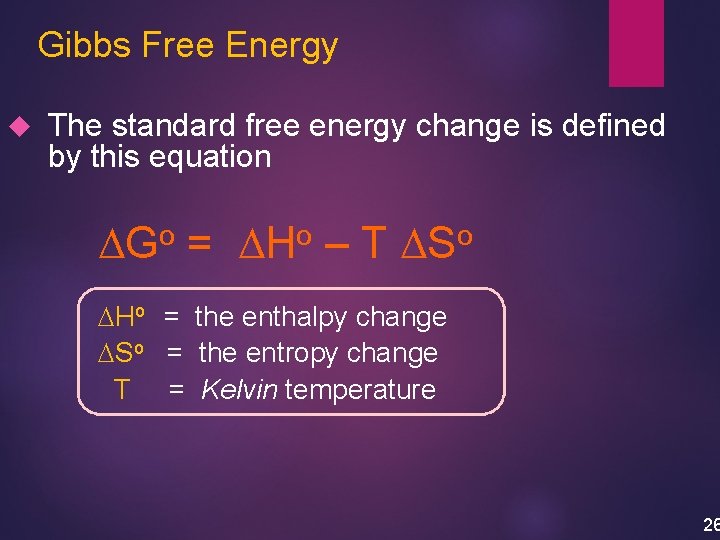
Gibbs Free Energy The standard free energy change is defined by this equation DGo = DHo – T DSo DHo = the enthalpy change DSo = the entropy change T = Kelvin temperature 26

Gibbs Free Energy Chem Rxn spontaneous if DGo <0 So let’s look at possible combinations of DHo and TDSo for free energy change: DGo = DHo – TDSo 27

When is Gibbs Free Energy (-) ? ! DGo = DHo – TDSo o DG Hot & m. ESs. Y! negative so DHospontaneous! <0 and DSo >0 DGo positive so NONspontaneous! Cold & Tidy DHo >0 and DSo <0 28
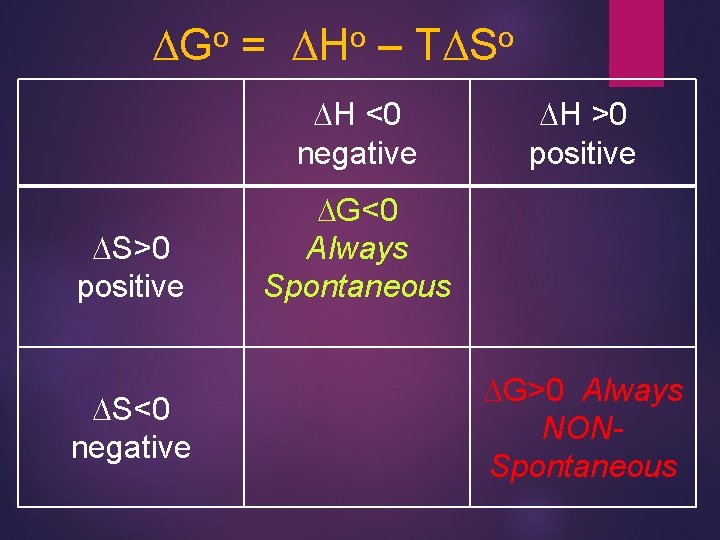
o DG = o DH – o TDS DH <0 negative DS>0 positive DS<0 negative DH >0 positive DG<0 Always Spontaneous DG>0 Always NONSpontaneous
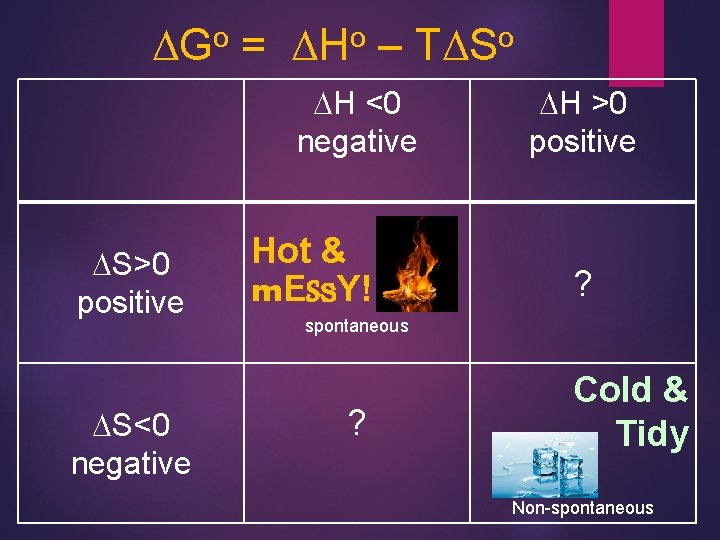
o DG = o DH – o TDS DH <0 negative DS>0 positive DS<0 negative Hot & m. ESs. Y! DH >0 positive ? spontaneous ? Cold & Tidy Non-spontaneous
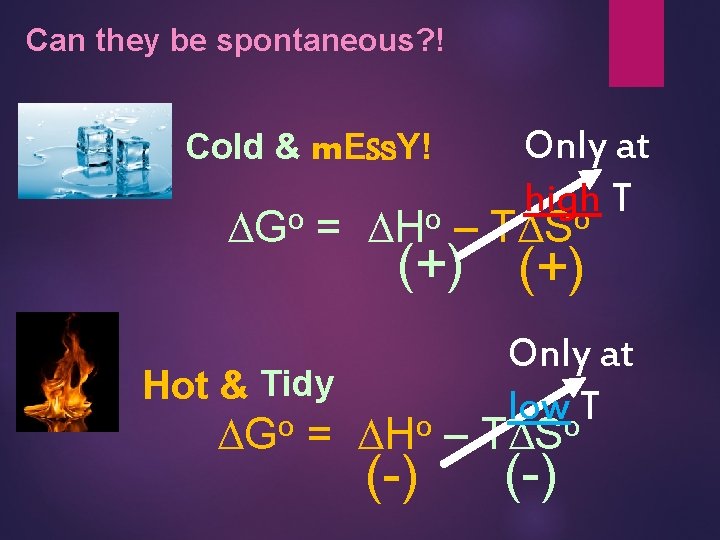
Can they be spontaneous? ! Only at high T DGo = DHo – TDSo Cold & m. ESs. Y! (+) Only at Hot & Tidy low T o o o DG = DH – TDS (-)
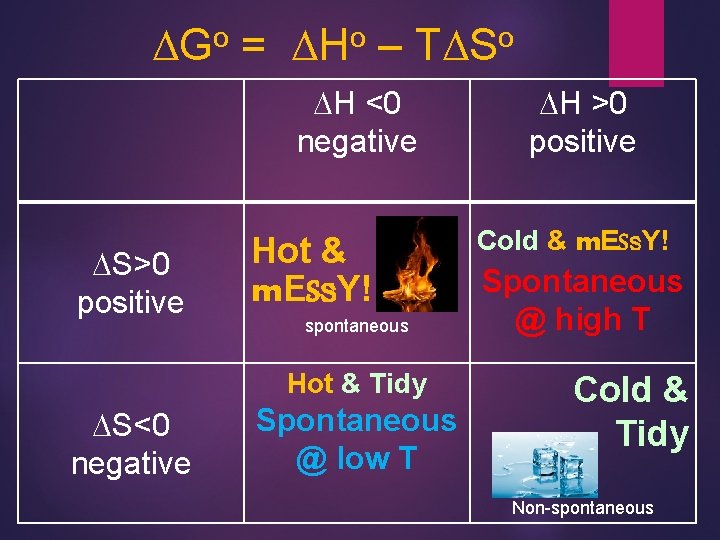
o DG = o DH – o TDS DH <0 negative DS>0 positive Hot & m. ESs. Y! spontaneous Hot & Tidy DS<0 negative Spontaneous @ low T DH >0 positive Cold & m. ESs. Y! Spontaneous @ high T Cold & Tidy Non-spontaneous
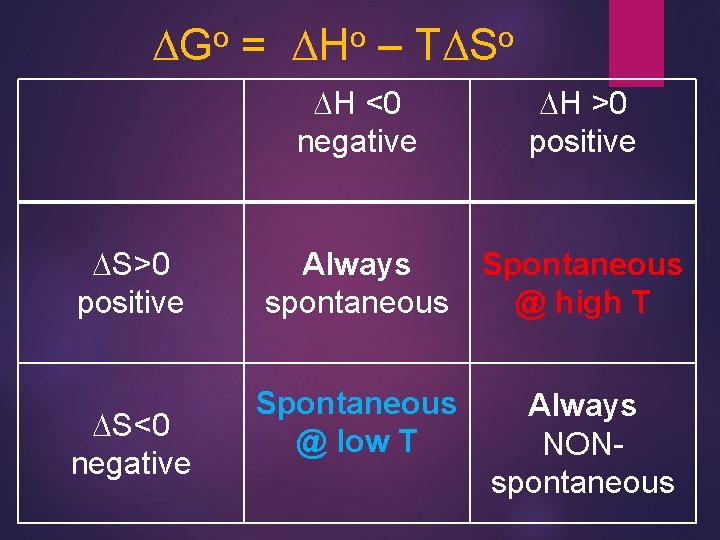
o DG DS>0 positive DS<0 negative = o DH – o TDS DH <0 negative DH >0 positive Always spontaneous Spontaneous @ high T Spontaneous @ low T Always NONspontaneous

Free Energy Problem 1 A certain chemical reaction is exothermic with a standard enthalpy of - 400 k. J mol-1. The entropy change for this reaction is +44 J mol-1 K-1. Calculate the free energy change for this reaction at 25 o. C. Is the reaction spontaneous? Solution Convert the entropy value 44 J ml-1 K-1 = 0. 044 k. J mol-1 K-1 DG = - 400 k. J mol-1 – (298 K)(0. 044 k. J mol-1 K-1) DG = - 400 k. J mol-1 – 13. 1 k. J mol-1 DG = - 413. 1 k. J mol-1. DG is negative spontaneous! Note. Because DH <0 and DS >0, this reaction is spontaneous at all temperatures. 34
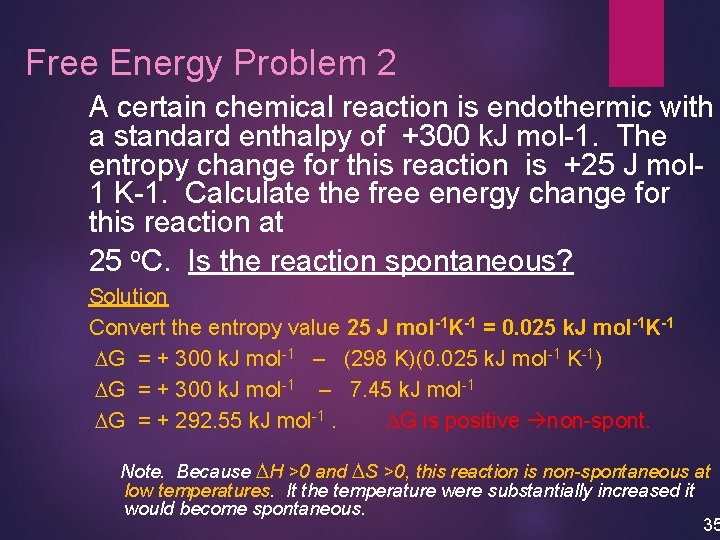
Free Energy Problem 2 A certain chemical reaction is endothermic with a standard enthalpy of +300 k. J mol-1. The entropy change for this reaction is +25 J mol 1 K-1. Calculate the free energy change for this reaction at 25 o. C. Is the reaction spontaneous? Solution Convert the entropy value 25 J mol-1 K-1 = 0. 025 k. J mol-1 K-1 DG = + 300 k. J mol-1 – (298 K)(0. 025 k. J mol-1 K-1) DG = + 300 k. J mol-1 – 7. 45 k. J mol-1 DG = + 292. 55 k. J mol-1. DG is positive non-spont. Note. Because DH >0 and DS >0, this reaction is non-spontaneous at low temperatures. It the temperature were substantially increased it would become spontaneous. 35
- Slides: 35