Thermodynamic Modelling Thermodynamic modelling at the National Physical
















- Slides: 64

Thermodynamic Modelling Thermodynamic modelling at the National Physical Laboratory Hugh Davies 8 March 2002

Content § National Physical Laboratory § NPL Materials Centre § Materials Processing Team § Thermodynamics and Process Modelling Group § MTDATA and the calculation of phase equilibria Models, Materials and Databases § Tour of Modules § Data assessment § Conclusion §

What We Are NPL is the UK’s National Standards Laboratory for physical measurements

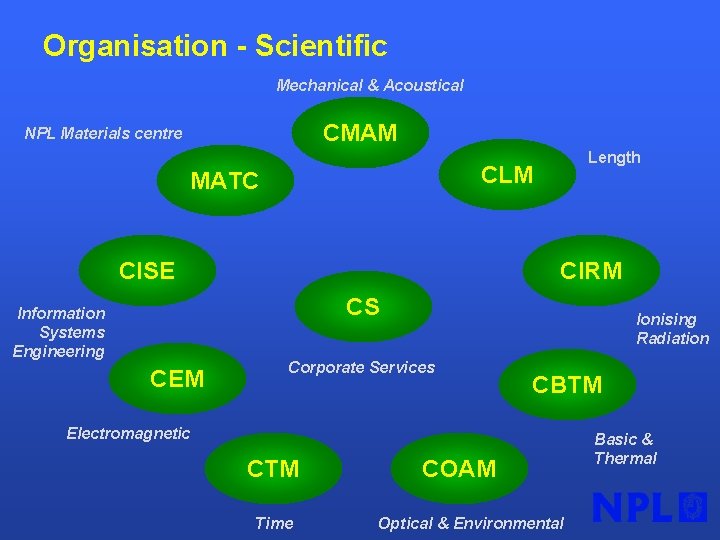
Organisation - Scientific Mechanical & Acoustical CMAM NPL Materials centre Length CLM MATC CISE CIRM CS Information Systems Engineering CEM Ionising Radiation Corporate Services CBTM Electromagnetic CTM Time COAM Optical & Environmental Basic & Thermal

Who are we? u NPL Materials Centre (MATC) is one of the world’s leading materials research laboratories u We are the largest scientific centre at the National Physical Laboratory in Teddington u We employ over 90 scientists working on a wide range of materials measurement areas. Currently, work is divided into four areas: Measurements for materials processing & manufacturing u Characterisation & performance of materials u Surface properties & engineering u Materials systems & components, design, modelling and databases u

Ceramics Surfaces & High temperature Interfaces Oxidation Residual Stress Corrosion Liquid Metal Processing Polymers Composites Life Prediction

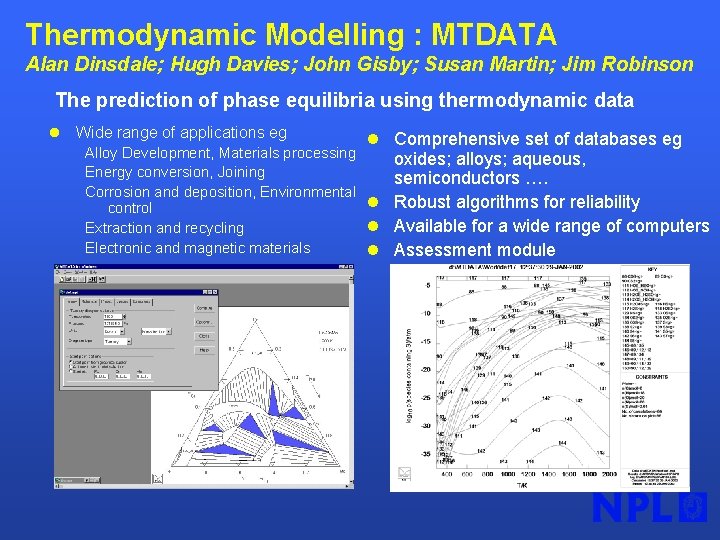
Thermodynamic Modelling : MTDATA Alan Dinsdale; Hugh Davies; John Gisby; Susan Martin; Jim Robinson The prediction of phase equilibria using thermodynamic data l Wide range of applications eg l Comprehensive set of databases eg Alloy Development, Materials processing Energy conversion, Joining Corrosion and deposition, Environmental l control l Extraction and recycling Electronic and magnetic materials l oxides; alloys; aqueous, semiconductors …. Robust algorithms for reliability Available for a wide range of computers Assessment module

Thermodynamic Modelling: Engineering Toolkits Alan Dinsdale; Hugh Davies; John Gisby; Susan Martin; Jim Robinson The use of predictive materials chemistry for process simulation and control l MTDATA Application Programming Interface for user written software l Api is self documenting for easy maintenance l Provides link to third party commercial packages eg CFx, Fluent, Physica, MAGMASoft l Custom built engineering toolkits for specific industries eg lighting applications, casting of alloys

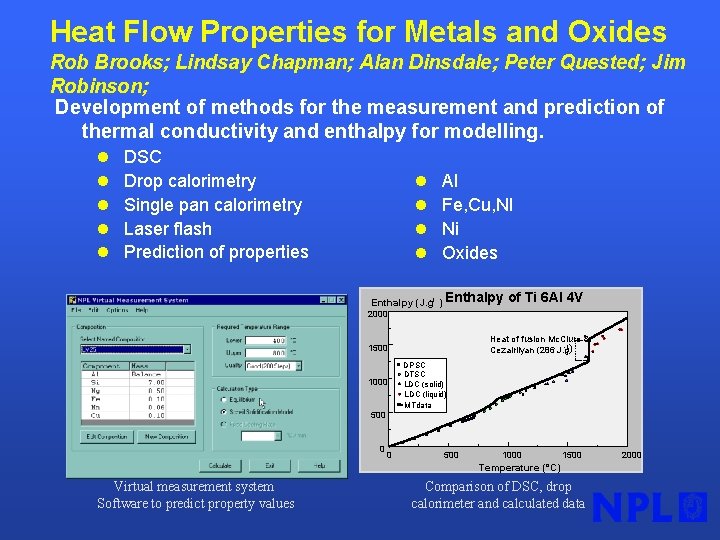
Heat Flow Properties for Metals and Oxides Rob Brooks; Lindsay Chapman; Alan Dinsdale; Peter Quested; Jim Robinson; Development of methods for the measurement and prediction of thermal conductivity and enthalpy for modelling. l l l DSC Drop calorimetry Single pan calorimetry Laser flash Prediction of properties l l Al Fe, Cu, NI Ni Oxides Enthalpy (J. g-1 ) Enthalpy of Ti 6 Al 4 V 2000 Heat of fusion Mc. Clure & -1 ) Cezairliyan (286 J. g 1500 1000 DPSC DTSC LDC (solid) LDC (liquid) MTdata 500 0 0 500 1000 1500 Temperature (°C) Virtual measurement system Software to predict property values Comparison of DSC, drop calorimeter and calculated data 2000

Thermodynamic modelling using MTDATA – This software/data package is used to predict the complex chemistry in multicomponent multiphase systems, using critically assessed thermodynamic data. It is used by companies and universities worldwide for such diverse applications as continuous casting of steel, lamp chemistry, pollution control and pyrometallurgical extraction.

What is MTDATA ? Designed to calculate phase/chemical equilibria with maximum ease and reliability § Interactive § User application programming § Despite reports to the contrary MTDATA does not work like other comparable programs § A true Gibbs energy minimisation § A non-linear optimisation problem with linear constraints § NPL Numerical Optimisation Software Library § No initial guess required § Mathematical guarantee that G reduces each time it is evaluated § Result can be proven to be a solution to the posed problem § Very high reliability §

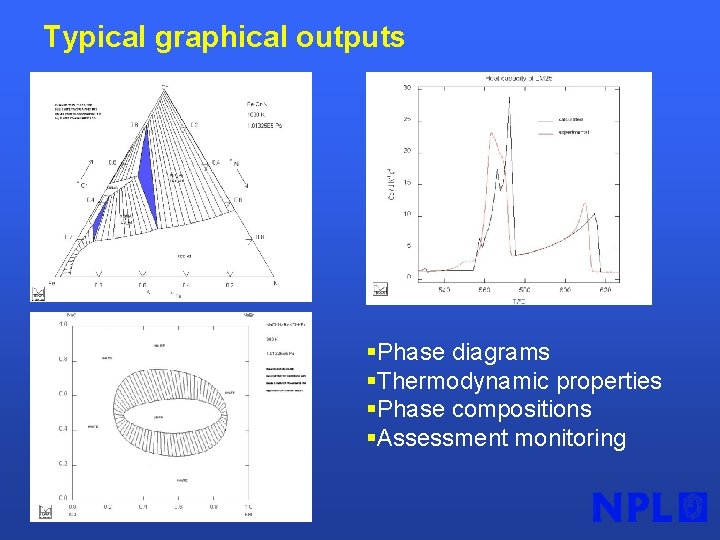
Typical graphical outputs §Phase diagrams §Thermodynamic properties §Phase compositions §Assessment monitoring

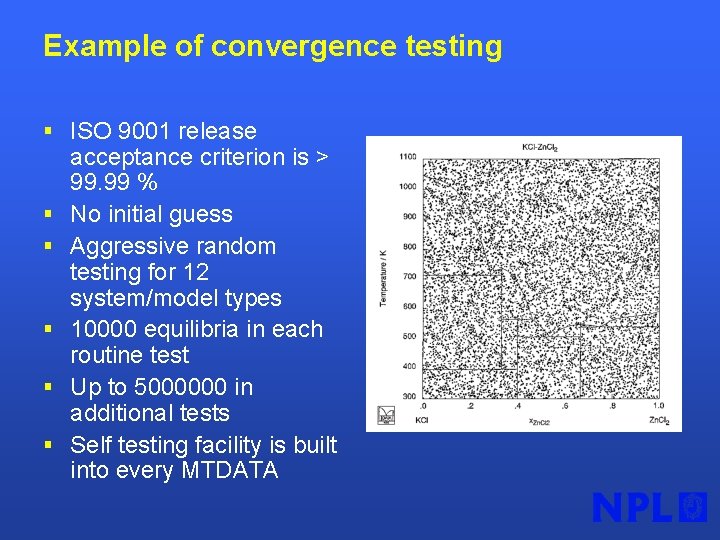
Example of convergence testing § ISO 9001 release § § § acceptance criterion is > 99. 99 % No initial guess Aggressive random testing for 12 system/model types 10000 equilibria in each routine test Up to 5000000 in additional tests Self testing facility is built into every MTDATA

Too good to be true ? § What can go wrong - not much § near 100% likelihood of convergence § slow convergence could be a problem § Miscibility gaps and systems with “ordering” § in some dimension the Gibbs energy appears to have multiple minima § heuristic methods give good reliability § MTDATA is very strong on calculations in systems with miscibility gaps and phases whose Gibbs energy is not an explicit function of system composition

Models and Materials § Data for a phase consists of: § Unary G as f(T, P) possibly magnetic with one or more sublattices § Gex as f(T, P, x), typical models: Redlich Kister § Quasi chemical § Modified Wagner dilute § Modified Pitzer § § § ternary or reciprocal interactions To model a wide range of mostly inorganic materials § Alloys § Semi-conductors § Oxides § Salts § Aqueous solution § Gas phase and stoichiometric solids § Others (eg Polymer solutions, Organic vapours and Bio systems)

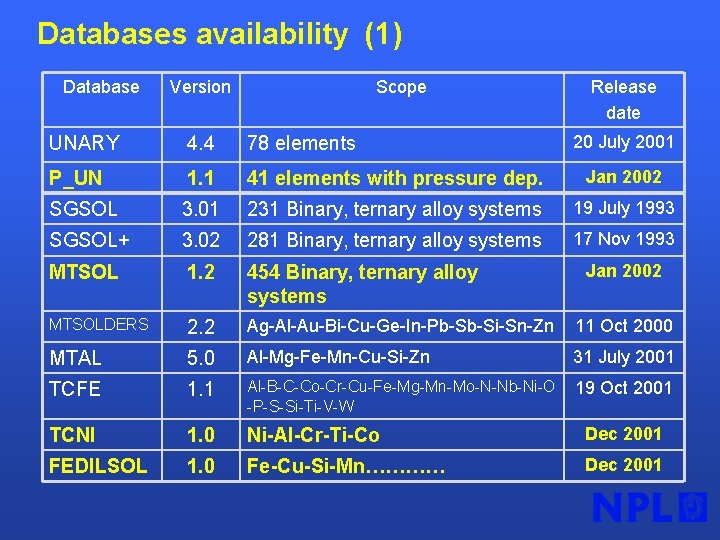
Databases availability (1) Database Version Scope Release date 20 July 2001 UNARY 4. 4 78 elements P_UN 1. 1 41 elements with pressure dep. Jan 2002 SGSOL 3. 01 231 Binary, ternary alloy systems 19 July 1993 SGSOL+ 3. 02 281 Binary, ternary alloy systems 17 Nov 1993 MTSOL 1. 2 454 Binary, ternary alloy systems MTSOLDERS 2. 2 Ag-Al-Au-Bi-Cu-Ge-In-Pb-Sb-Si-Sn-Zn 11 Oct 2000 MTAL 5. 0 Al-Mg-Fe-Mn-Cu-Si-Zn 31 July 2001 TCFE 1. 1 Al-B-C-Co-Cr-Cu-Fe-Mg-Mn-Mo-N-Nb-Ni-O -P-S-Si-Ti-V-W 19 Oct 2001 TCNI 1. 0 Ni-Al-Cr-Ti-Co Dec 2001 FEDILSOL 1. 0 Fe-Cu-Si-Mn………… Dec 2001 Jan 2002

Databases availability (2) Database Version Scope Release date MTSEMI 1. 1 Al-As-Ga-In-P-Sb Feb 2002 MTOX 4. 4 Li 2 O-Na 2 O-K 2 O-Ca. O-Mg. O-Ni. O-Pb. O-Zn. OCu-Cr-Fe-Mn-O-Al 2 O 3 -B 2 O 3 -Zr. O 2 -Si. O 2 -S Jan 2002 NPLOX 1 1. 0 Ca. O-Al 2 O 3 -Si. O 2 26 Apr 1996 NPLOX 2 1. 0 Ca. O-Mg. O-Fe-O-Al 2 O 3 -Si. O 2 11 Jan 2001 NPLOX 3 1. 0 Na 2 O-Ca. O-Mg. O-Fe-O-Al 2 O 3 -Si. O 2 26 Jun 2001 NPLCORR 2. 0 Al-Cr-Fe-Mn-Ni-Si-O IRSID 1. 0 Ca. O-Mg. O-Fe. O-Mn. O-Al 2 O 3 -Si. O 2 QUASI 1. 0 Selected systems SALTS 2. 0 147 binary or reciprocal systems MTAQ 2. 1 ~ 30 binary systems Dec 2001 17 Dec 1997 Mar 2002 28 Nov 2001 Dec 2001

Databases availability (3) Database Version Scope Release date AQDATA 1. 2 471 aqueous substances 20 Sep 2000 HOTAQ 1. 0 Fe, Cr, Ni, C, S, H, O, /- 26 Mar 1993 MTCHVAL 1. 2 Dilute aqueous data for room temperature nuclear applications 3 Nov 1999 MTSPCRT 1. 3 Dilute aqueous data for geochemical systems spanning a range of temperatures 3 Nov 1999 SGSUB 10. 0 4152 gaseous and condensed substances 9 May 2001 SGORG 10. 0 150 organic substances (C>3) 9 May 2001 MTNUCL 1. 0 Oxides, alloys, salts, aqueous, gaseous Mar 2002

Typical output from UNARY database

Typical output from P_UN database (1)

Typical output from P_UN database (2)

Typical output from TCFE database

Typical output from MTSOLDERS database Ag-Cu-Pb-Sn system

Typical output from MTSOL database

Typical output from MTAL database

Typical output from SGSUB database

Typical output from NPLOX 2 database

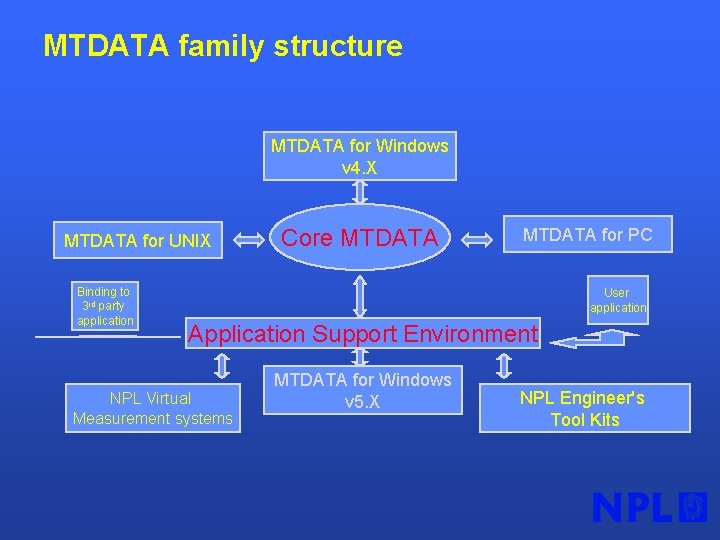
MTDATA family structure MTDATA for Windows v 4. X MTDATA for UNIX Binding to 3 rd party application Core MTDATA for PC User application Application Support Environment NPL Virtual Measurement systems MTDATA for Windows v 5. X NPL Engineer's Tool Kits

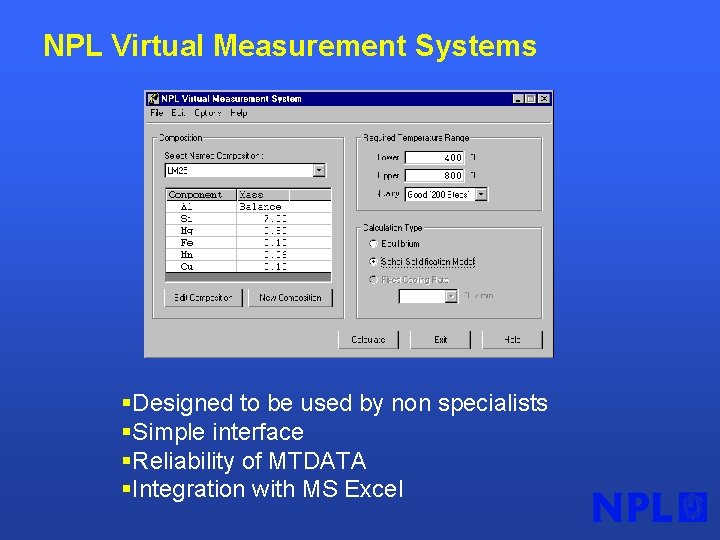
NPL Virtual Measurement Systems §Designed to be used by non specialists §Simple interface §Reliability of MTDATA §Integration with MS Excel

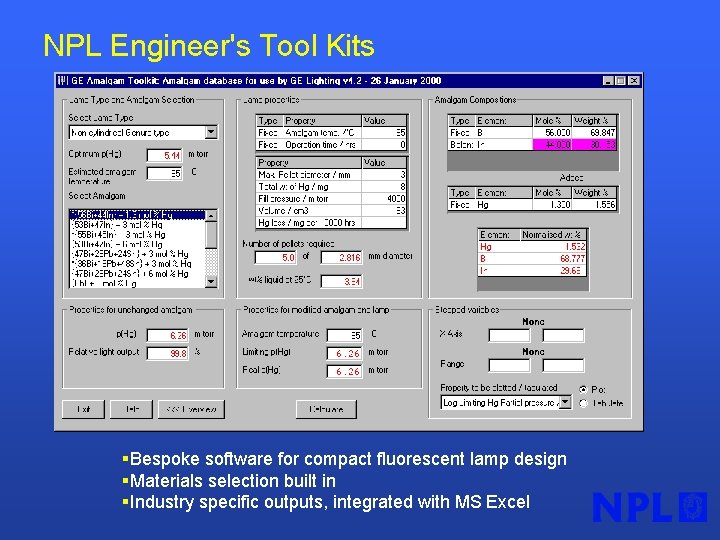
NPL Engineer's Tool Kits §Bespoke software for compact fluorescent lamp design §Materials selection built in §Industry specific outputs, integrated with MS Excel

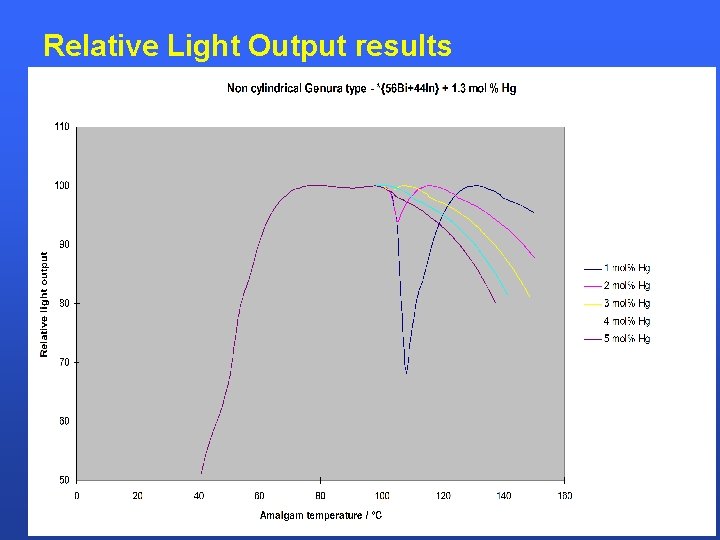
Relative Light Output results

EHECATL – General MTDATA/Fluent binding

Aqueous Chemistry(1) U-H 2 O system

Aqueous Chemistry(2) H 2 O-Am(NO 3)3 -EDTA

Ag to Pb 0. 5 Zn 0. 5 isopleth including gas phase

Ag to Pb 0. 5 Zn 0. 5 isopleth including gas phase

Ag to Pb 0. 5 Zn 0. 5 P-x diagram at 800 K

LM 25 equilibrium solidification

LM 25 “Scheil” solidification (1)

LM 25 “Scheil” solidification (2)

Metal – metal halide phase equilibria

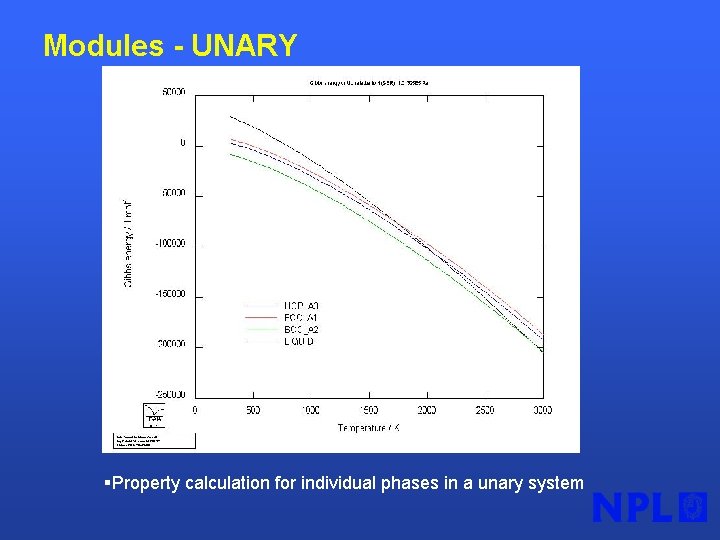
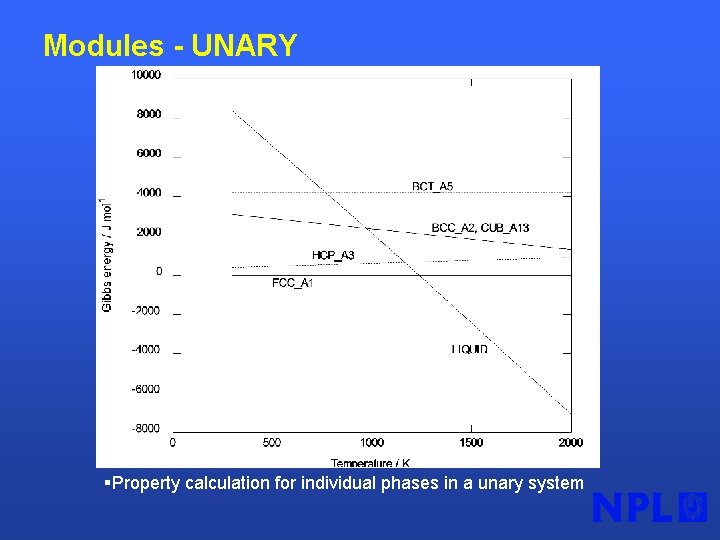
Modules - UNARY §Property calculation for individual phases in a unary system

Modules - UNARY §Property calculation for individual phases in a unary system

Modules - UNARY §Property calculation for individual phases in a unary system

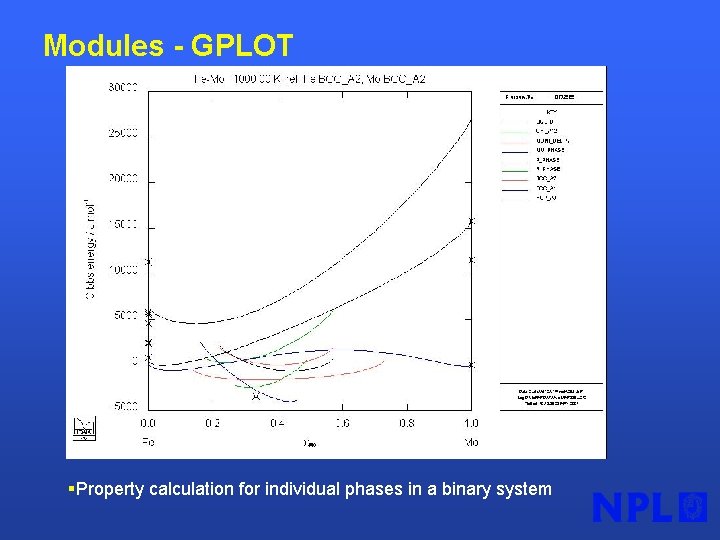
Modules - GPLOT §Property calculation for individual phases in a binary system

Modules - GPLOT §Property calculation for individual phases in a binary system

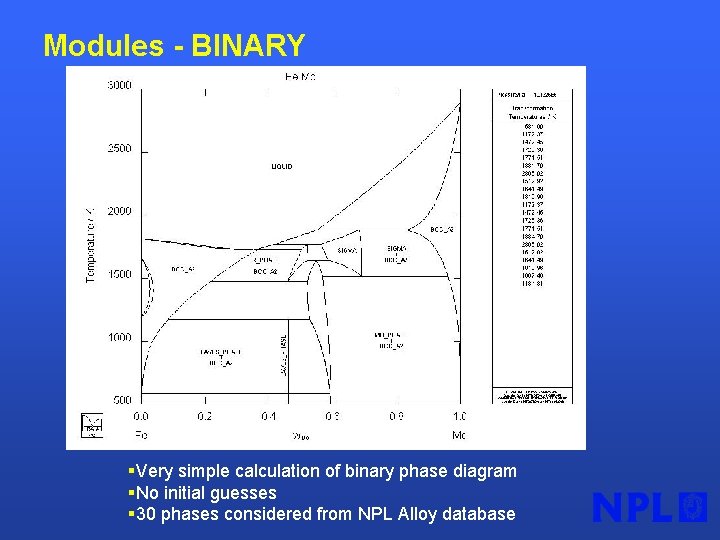
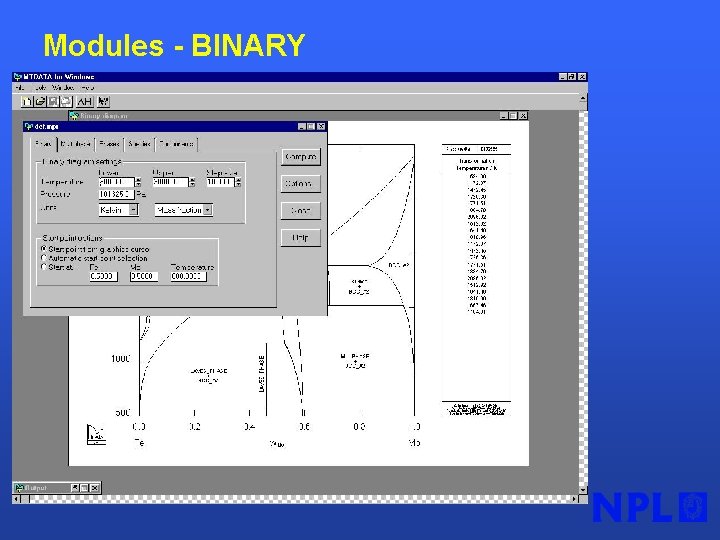
Modules - BINARY §Very simple calculation of binary phase diagram §No initial guesses § 30 phases considered from NPL Alloy database

Modules - BINARY §Very simple calculation of binary phase diagram §No initial guesses § 30 phases considered from NPL Alloy database

Modules - BINARY

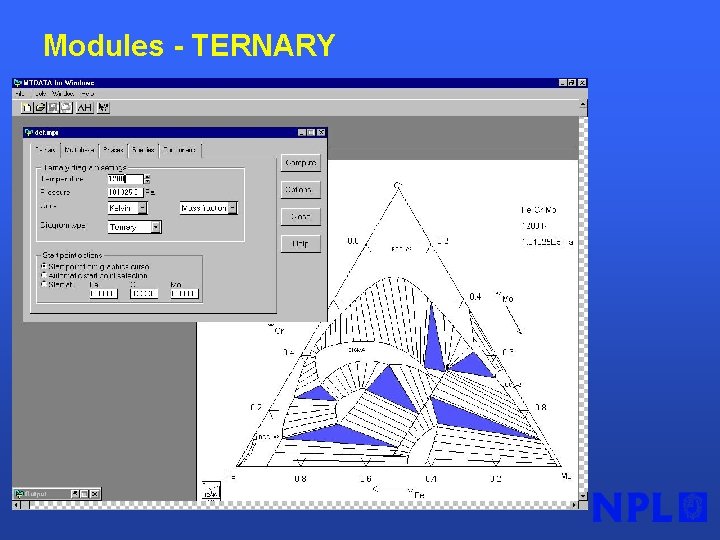
Modules - TERNARY §Very simple calculation of isothermal ternary or reciprocal phase diagrams §No initial guesses §Interactive labelling § 24 phases considered from NPL Oxide database

Modules - TERNARY §Very simple calculation of isothermal ternary or reciprocal phase diagrams §No initial guesses §Interactive labelling

Modules - TERNARY

Modules - MULTIPHASE §Interactive interface to the core equilibrium algorithm §Fixed T, P(V), x or variable in many ways §Open systems §Activity, composition, enthalpy constraints and Para equilibrium

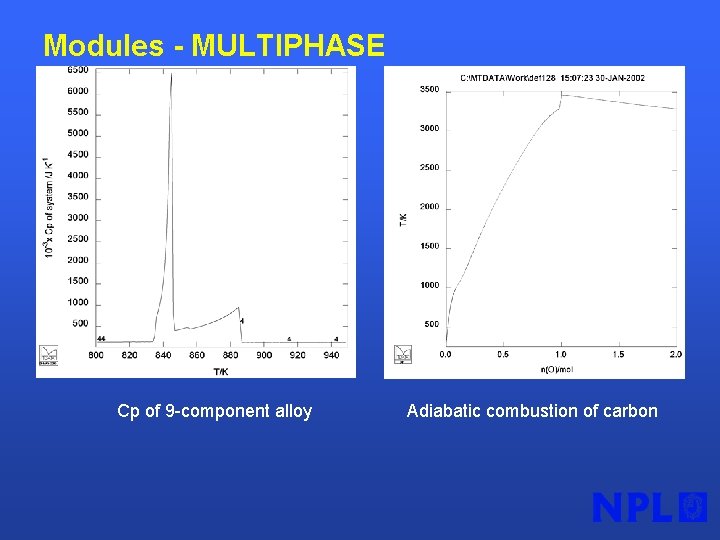
Modules - MULTIPHASE Cp of 9 -component alloy Adiabatic combustion of carbon

Modules - MULTIPHASE

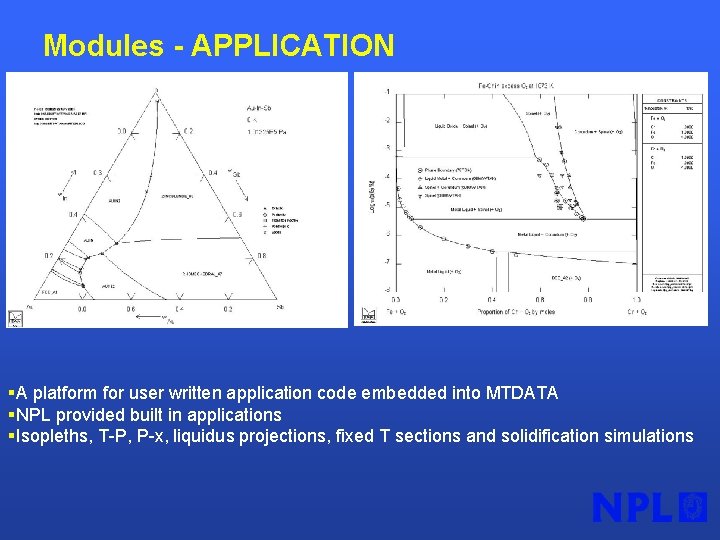
Modules - APPLICATION §A platform for user written application code embedded into MTDATA §NPL provided built in applications §Isopleths, T-P, P-x, liquidus projections, fixed T sections and solidification simulations

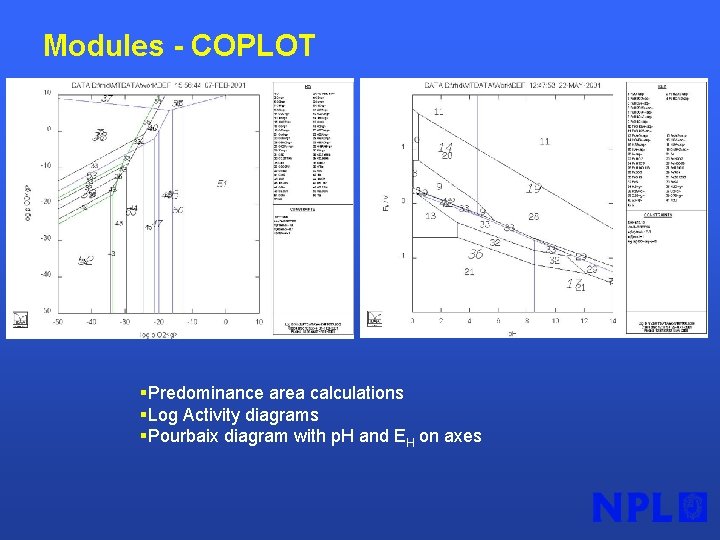
Modules - COPLOT §Predominance area calculations §Log Activity diagrams §Pourbaix diagram with p. H and EH on axes

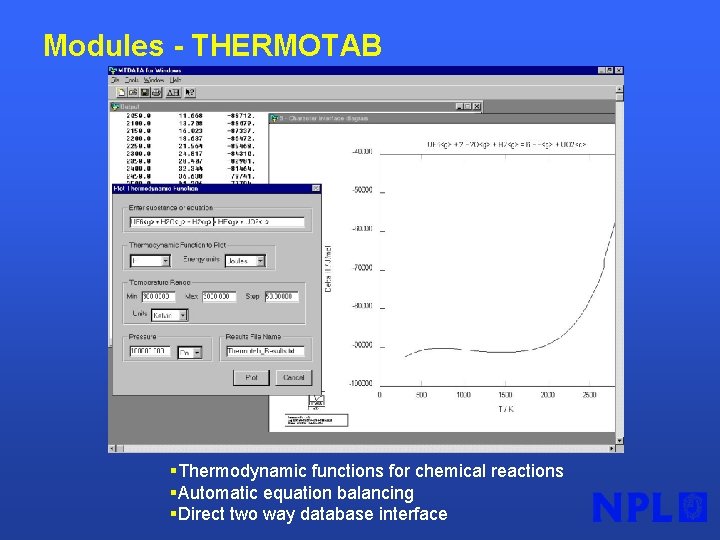
Modules - THERMOTAB §Thermodynamic functions for chemical reactions §Automatic equation balancing §Direct two way database interface

Data Assessment § Completely general framework for data assessment § MTDATA command language allows virtually any experimental information to be included § No practical limit to the size of system being assessed § Any thermodynamic model parameter can be included in the assessment process § Real-time graphical monitoring of objective function and parameter values § Extensive graphical results reporting § Integration with the rest of MTDATA

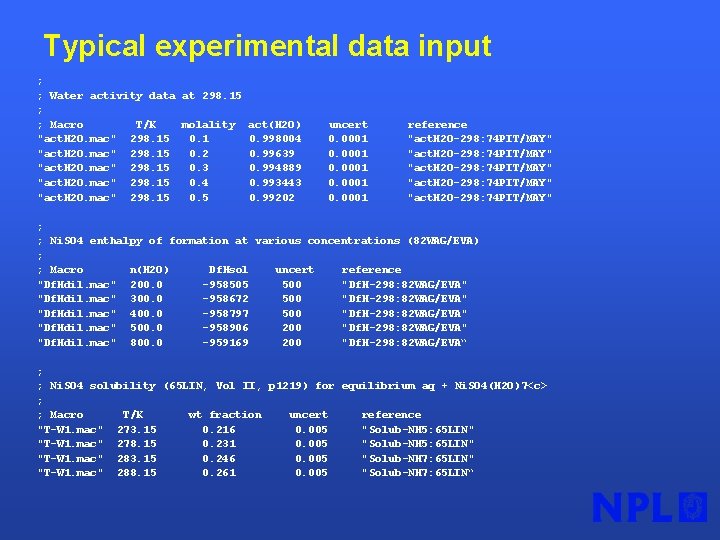
Typical experimental data input ; ; Water activity data at 298. 15 ; ; Macro T/K molality "act. H 2 O. mac" 298. 15 0. 1 "act. H 2 O. mac" 298. 15 0. 2 "act. H 2 O. mac" 298. 15 0. 3 "act. H 2 O. mac" 298. 15 0. 4 "act. H 2 O. mac" 298. 15 0. 5 act(H 2 O) 0. 998004 0. 99639 0. 994889 0. 993443 0. 99202 uncert 0. 0001 reference "act. H 2 O-298: 74 PIT/MAY" ; ; Ni. SO 4 enthalpy of formation at various concentrations (82 WAG/EVA) ; ; Macro n(H 2 O) Df. Hsol uncert reference "Df. Hdil. mac" 200. 0 -958505 500 "Df. H-298: 82 WAG/EVA" "Df. Hdil. mac" 300. 0 -958672 500 "Df. H-298: 82 WAG/EVA" "Df. Hdil. mac" 400. 0 -958797 500 "Df. H-298: 82 WAG/EVA" "Df. Hdil. mac" 500. 0 -958906 200 "Df. H-298: 82 WAG/EVA" "Df. Hdil. mac" 800. 0 -959169 200 "Df. H-298: 82 WAG/EVA“ ; ; Ni. SO 4 solubility (65 LIN, Vol II, p 1219) for equilibrium aq + Ni. SO 4(H 2 O)7<c> ; ; Macro T/K wt fraction uncert reference "T-W 1. mac" 273. 15 0. 216 0. 005 "Solub-NH 5: 65 LIN" "T-W 1. mac" 278. 15 0. 231 0. 005 "Solub-NH 5: 65 LIN" "T-W 1. mac" 283. 15 0. 246 0. 005 "Solub-NH 7: 65 LIN" "T-W 1. mac" 288. 15 0. 261 0. 005 "Solub-NH 7: 65 LIN“

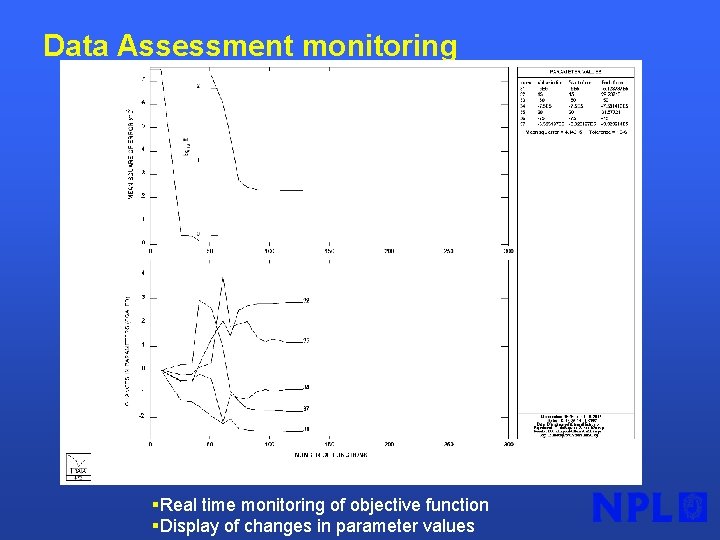
Data Assessment monitoring §Real time monitoring of objective function §Display of changes in parameter values

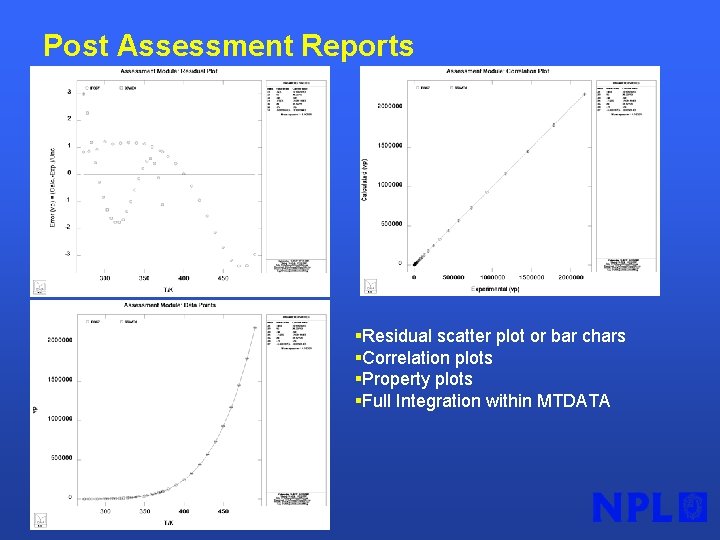
Post Assessment Reports §Residual scatter plot or bar chars §Correlation plots §Property plots §Full Integration within MTDATA

Conclusions § § § MTDATA is a very general tool for calculating phase and chemical equilibria with very high reliability and no need for initial guesses at the system/phase composition A wide range of models and databases allow application to a large number of different types of problem Complex user applications can be built using calls to MTDATA routines from users code or 3 rd party applications Produced by NPL the UK National Standards Laboratory Thanks to Alan Dinsdale, John Gisby, Jim Robinson, Susan Martin and Malcolm Rand Funding in part from DTI EID and all commercial users of MTDATA
