Thermochemistry The study of heat energy changes in










- Slides: 23

Thermochemistry • The study of heat energy changes in chemical reactions. HW 12 -1, #1 • Importance: 1. Safety considerations. KABOOM! Kabom link 2. Heat energy used for work. Examples: Burn methane to heat home. Burn gasoline to power car. Burn coal to produce electricity. Bozeman energy transformation

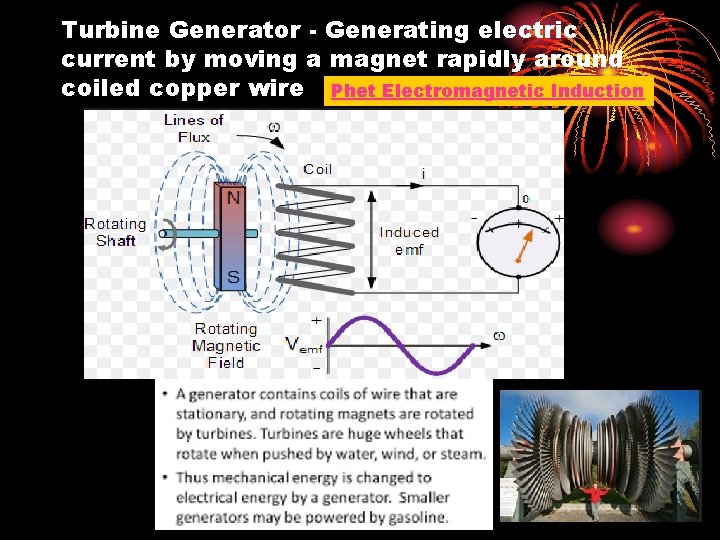
Turbine Generator - Generating electric current by moving a magnet rapidly around coiled copper wire Phet Electromagnetic Induction

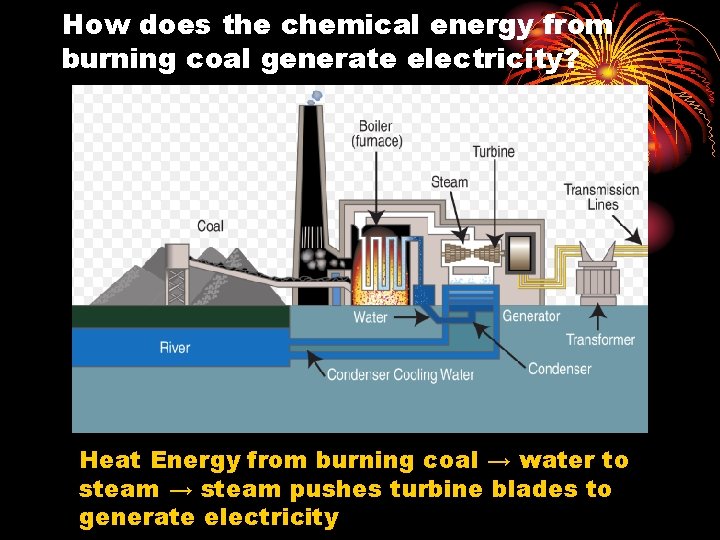
How does the chemical energy from burning coal generate electricity? Heat Energy from burning coal → water to steam → steam pushes turbine blades to generate electricity

What is Energy? • Energy = capacity to do work • Work = Force x distance • “working” defn of energy = capacity to make objects move

1 st Law of Thermodynamics • Energy can neither be created nor destroyed! Phet skateboard link • Energy can only be transformed from one form to another. • Also known as the Conservation of Energy Bozeman video link

How badly do you want the “Free Stuff? ” What could POTENTIALLY happen?

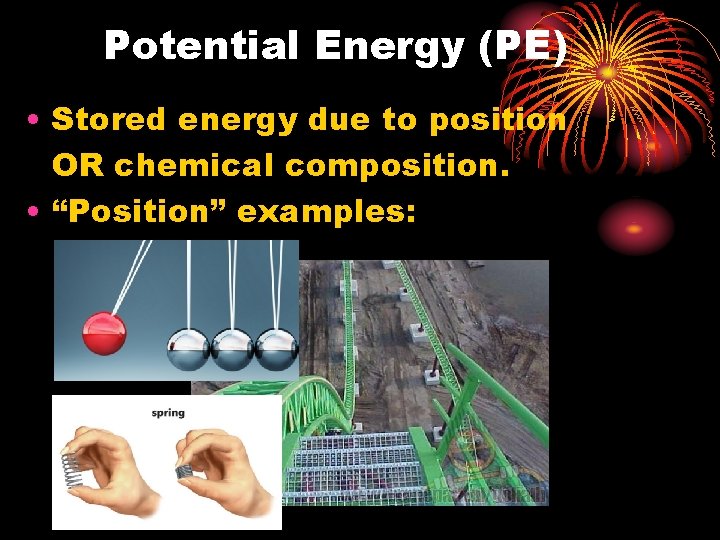
Potential Energy (PE) • Stored energy due to position OR chemical composition. • “Position” examples:

Potential energy transformed into. . .

Kinetic Energy(KE) • Energy of Motion BUNGEE JUMP LINK

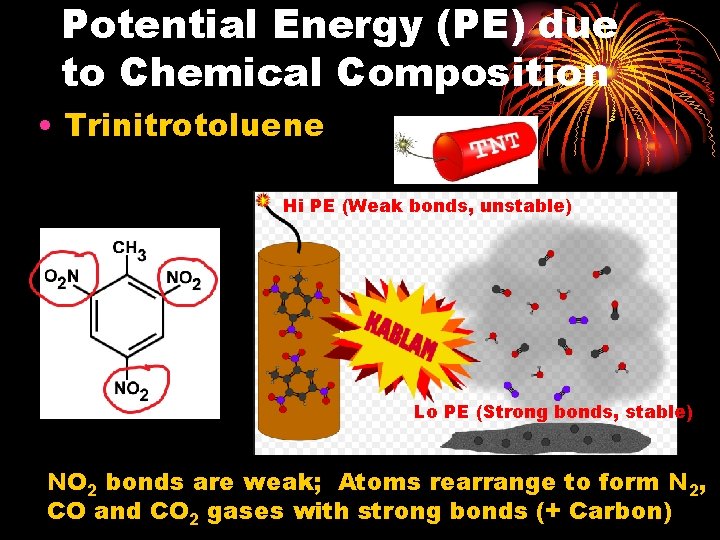
Potential Energy (PE) due to Chemical Composition • Trinitrotoluene Hi PE (Weak bonds, unstable) Lo PE (Strong bonds, stable) NO 2 bonds are weak; Atoms rearrange to form N 2, CO and CO 2 gases with strong bonds (+ Carbon)

Potential TO become. . .

Kinetic Energy (KE)- molecules with higher KE move faster Product Molecules have Lower PE but much Higher Kinetic energy molecules FIREBALL LINK

HW 12 -1, #2 Location PE UNITS KE UNITS TOTAL ENERGY 1 10 2 6 3 2 0 4 8 10 10 10

The Ethanol Cannon: Example of First Law in Action. . Converting Stored Potential Chemical Energy into Kinetic Energy!

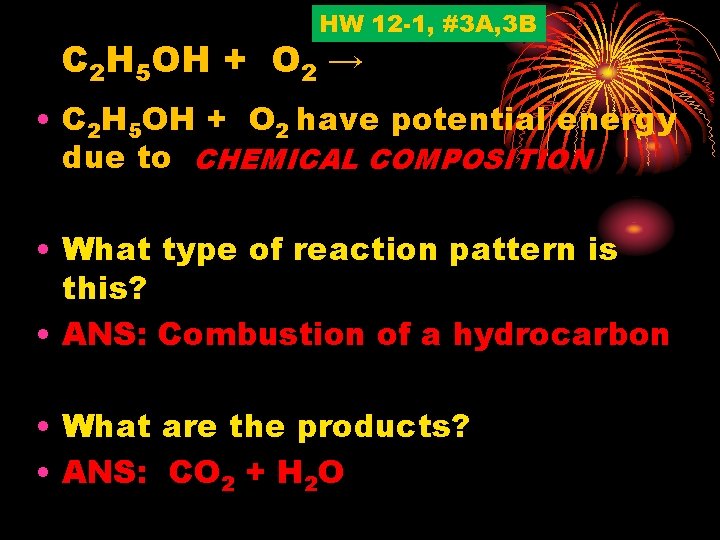
HW 12 -1, #3 A, 3 B C 2 H 5 OH + O 2 → • C 2 H 5 OH + O 2 have potential energy due to CHEMICAL COMPOSITION • What type of reaction pattern is this? • ANS: Combustion of a hydrocarbon • What are the products? • ANS: CO 2 + H 2 O

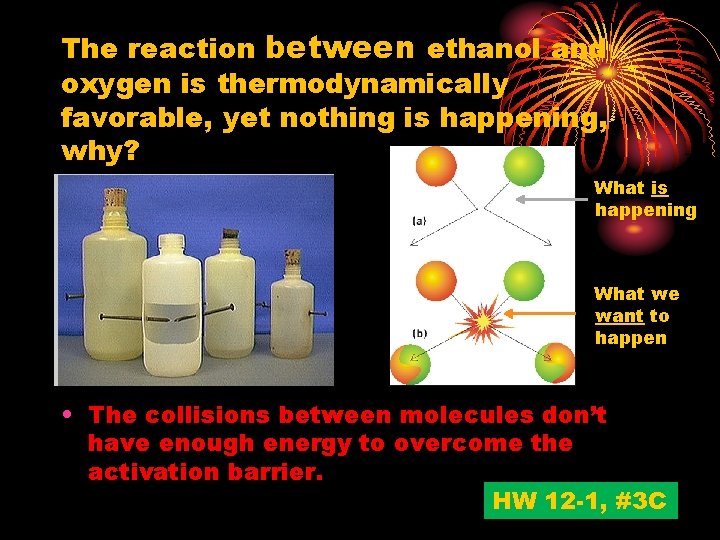
The reaction between ethanol and oxygen is thermodynamically favorable, yet nothing is happening, why? What is happening What we want to happen • The collisions between molecules don’t have enough energy to overcome the activation barrier. HW 12 -1, #3 C

Meet Mr. Tesla Coil! As electricity travels across the gap between the two nails it will heat the gas molecules past the Ea

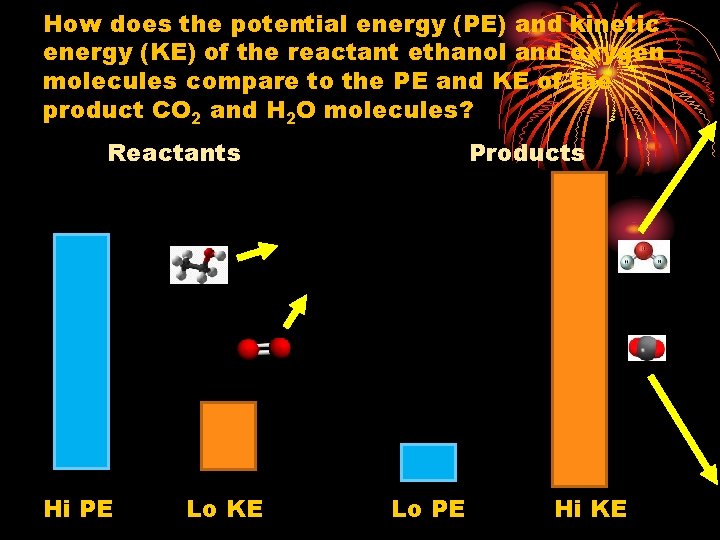
How does the potential energy (PE) and kinetic energy (KE) of the reactant ethanol and oxygen molecules compare to the PE and KE of the product CO 2 and H 2 O molecules? Reactants Hi PE Lo KE Products Lo PE Hi KE

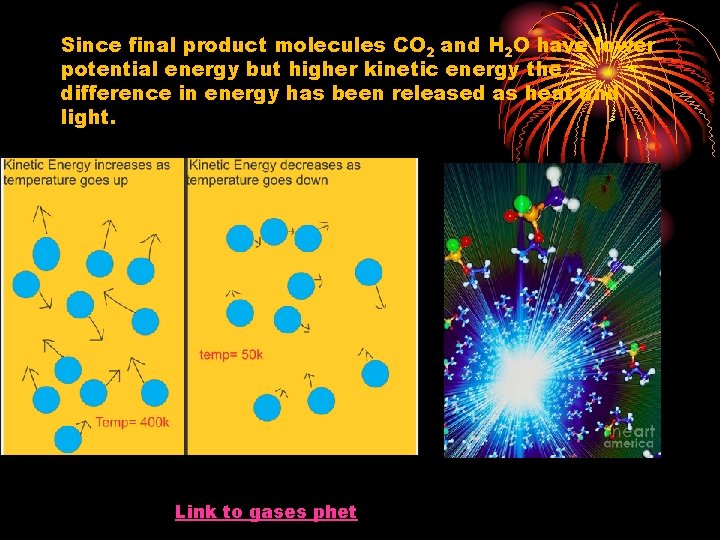
Since final product molecules CO 2 and H 2 O have lower potential energy but higher kinetic energy the difference in energy has been released as heat and light. Link to gases phet

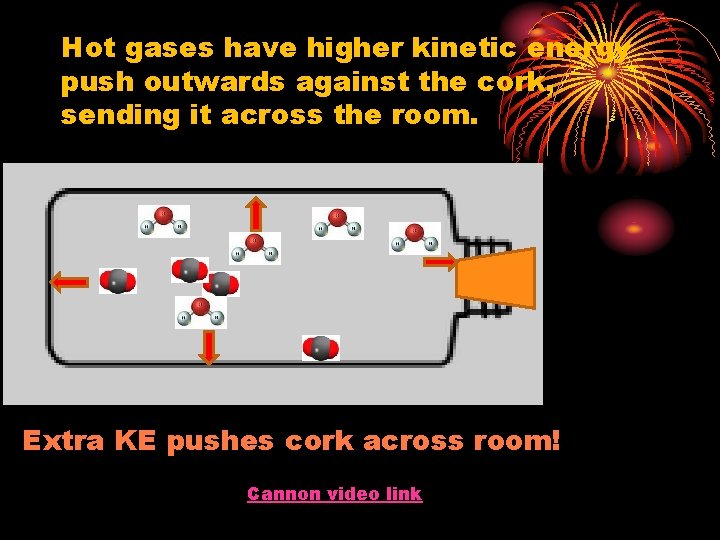
Hot gases have higher kinetic energy push outwards against the cork, sending it across the room. Extra KE pushes cork across room! Cannon video link

HW 12 -1, #3 D REACTANTS PE PE PE KE PRODUCTS KE PE KE KE

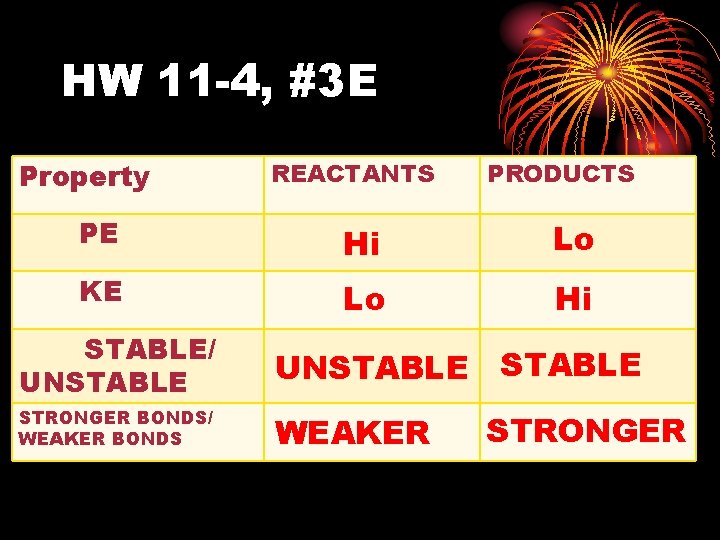
HW 11 -4, #3 E Property REACTANTS PRODUCTS PE Hi Lo KE Lo Hi STABLE/ UNSTABLE STRONGER BONDS/ WEAKER BONDS WEAKER STRONGER

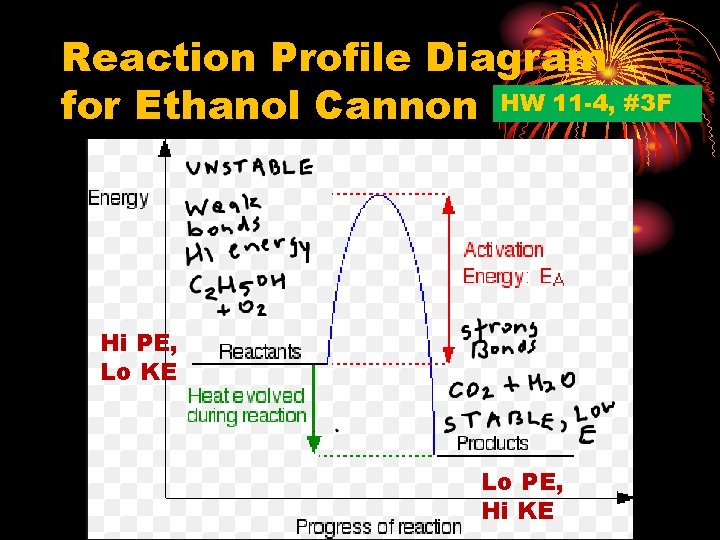
Reaction Profile Diagram for Ethanol Cannon HW 11 -4, #3 F Hi PE, Lo KE Lo PE, Hi KE
 Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of...
Thermochemistry is the study of... Thermochemistry is the study of
Thermochemistry is the study of Study of energy transformations
Study of energy transformations Termokimia adalah cabang ilmu kimia yang mempelajari ...
Termokimia adalah cabang ilmu kimia yang mempelajari ... Thermochemistry is the study of
Thermochemistry is the study of Q = m x cp x ∆t
Q = m x cp x ∆t Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry Elizabeth mulroney
Elizabeth mulroney Chemical change vs physical change
Chemical change vs physical change Thermal energy vs heat energy
Thermal energy vs heat energy Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Baking is a chemical change
Baking is a chemical change A researcher decides to study cognitive changes
A researcher decides to study cognitive changes Enthalpy change definition
Enthalpy change definition Energy changes
Energy changes Energy changes
Energy changes What is thermochemistry
What is thermochemistry Specific heat capacity
Specific heat capacity Specific latent heat formula
Specific latent heat formula Principle of cooking
Principle of cooking