Thermochemistry Second law of thermodynamics Ph D Halina











- Slides: 30

Thermochemistry. Second law of thermodynamics Ph. D. Halina Falfushynska

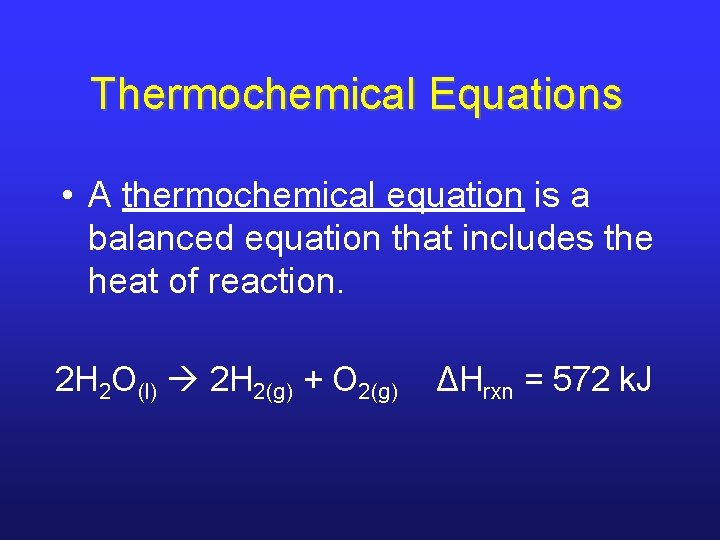
Thermochemical Equations • A thermochemical equation is a balanced equation that includes the heat of reaction. 2 H 2 O(l) 2 H 2(g) + O 2(g) ΔHrxn = 572 k. J

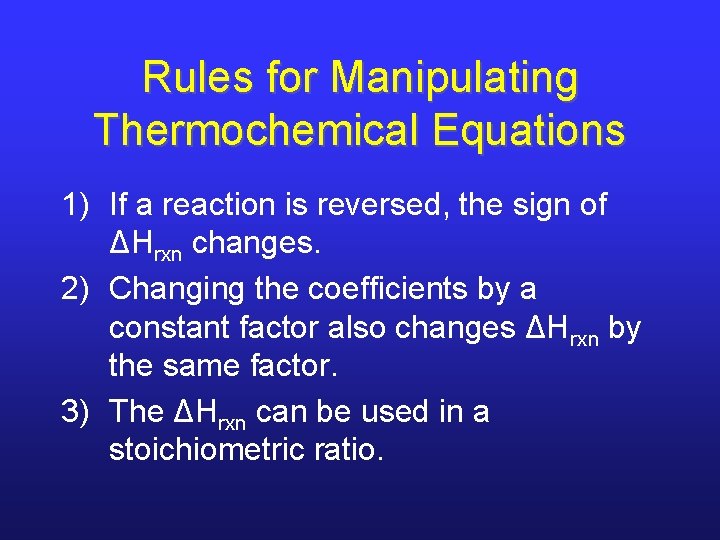
Rules for Manipulating Thermochemical Equations 1) If a reaction is reversed, the sign of ΔHrxn changes. 2) Changing the coefficients by a constant factor also changes ΔHrxn by the same factor. 3) The ΔHrxn can be used in a stoichiometric ratio.

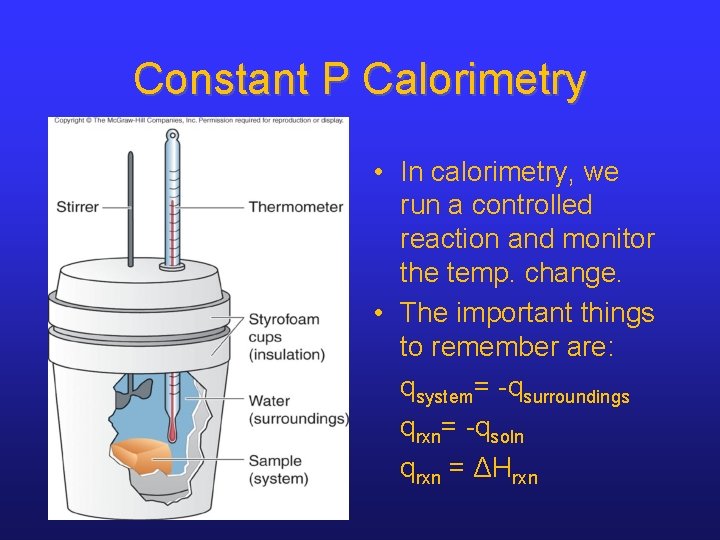
Constant P Calorimetry • In calorimetry, we run a controlled reaction and monitor the temp. change. • The important things to remember are: qsystem= -qsurroundings qrxn= -qsoln qrxn = ΔHrxn

Application and use of calorimetry in pharmaceutical development • characterize the stability of pharmaceutical compounds • Analysed small amounts of sample for screening relative reactivity • Should yield approximate relative reactivity rates under the various stressing conditions, which can be useful for planning how high of temperatures, and how much time would be required before enough degradation takes place to obtain reliable HPLC results

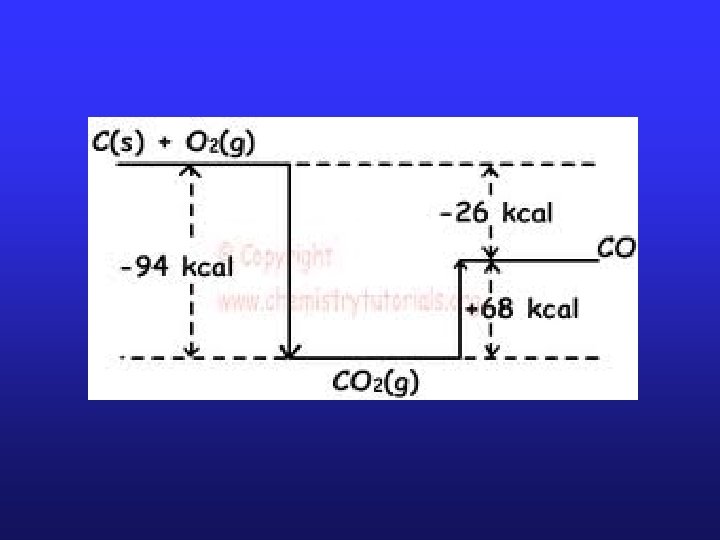
Hess’s Law • Some reactions are impossible or very difficult to carry out, yet we can still calculate what ΔH would be. • Hess’s Law: the enthalpy change of an overall process is the sum of the enthalpy changes of its individual steps • This means that you can break up a reaction into a series of smaller reactions that add up to the big reaction, and the ΔHrxn of the big reaction will be the sum of all the small reaction ΔHrxn’s.

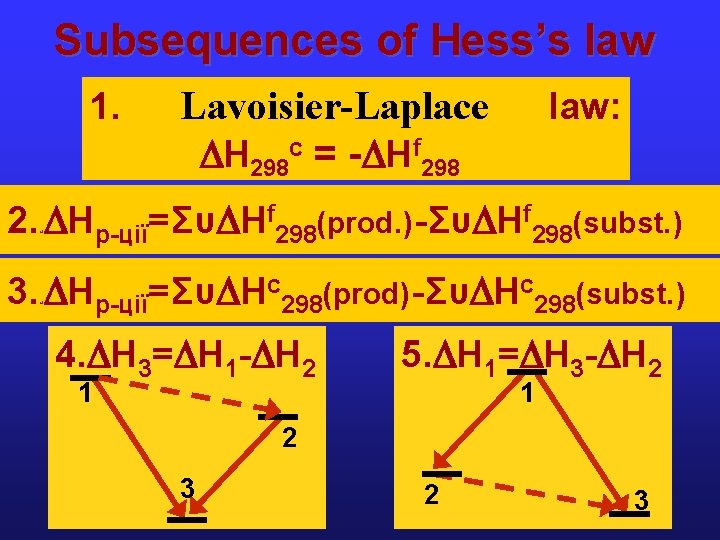
Subsequences of Hess’s law 1. Lavoisier-Laplace Н 298 c = - Нf 298 law: 2. Нр-ції= Συ Нf 298(prod. ) - Συ Нf 298(subst. ) м 3. Нр-ції= Συ Нс298(prod) - Συ Нс298(subst. ) м 4. Н 3= Н 1 - Н 2 1 5. Н 1= Н 3 - Н 2 1 2 3

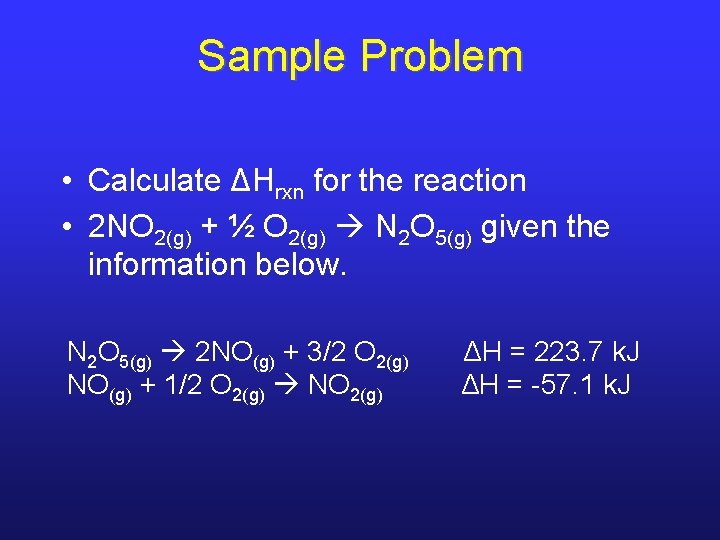
Sample Problem • Calculate ΔHrxn for the reaction • 2 NO 2(g) + ½ O 2(g) N 2 O 5(g) given the information below. N 2 O 5(g) 2 NO(g) + 3/2 O 2(g) ΔH = 223. 7 k. J NO(g) + 1/2 O 2(g) NO 2(g) ΔH = -57. 1 k. J

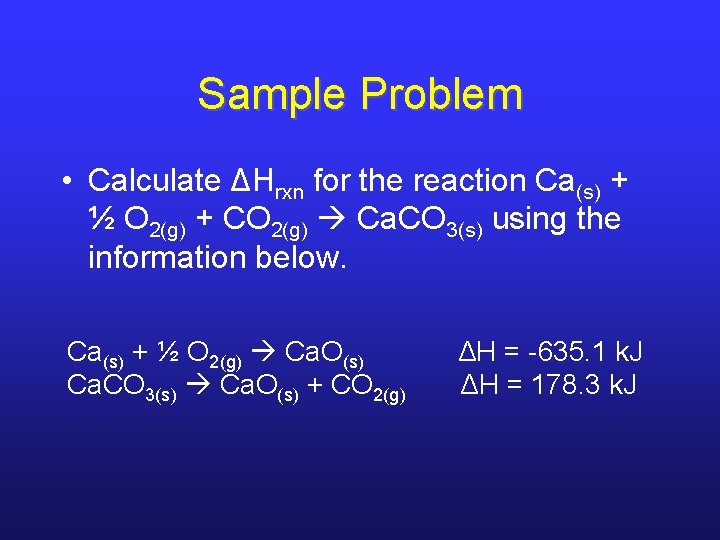
Sample Problem • Calculate ΔHrxn for the reaction Ca(s) + ½ O 2(g) + CO 2(g) Ca. CO 3(s) using the information below. Ca(s) + ½ O 2(g) Ca. O(s) ΔH = -635. 1 k. J Ca. CO 3(s) Ca. O(s) + CO 2(g) ΔH = 178. 3 k. J

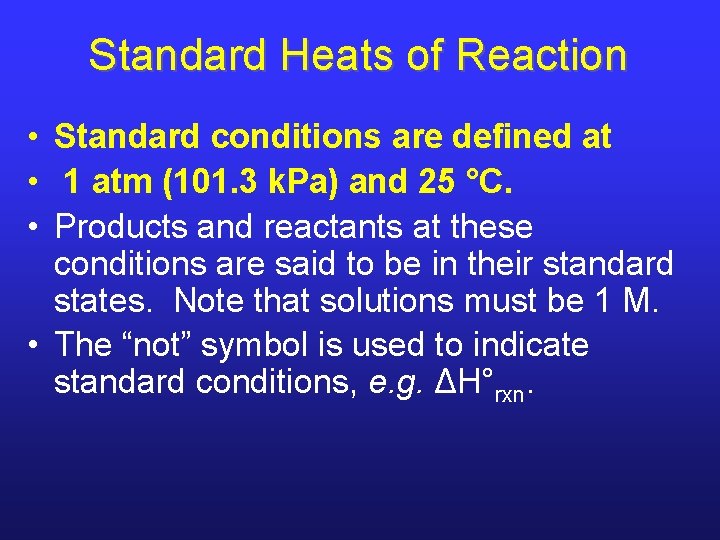
Standard Heats of Reaction • Standard conditions are defined at • 1 atm (101. 3 k. Pa) and 25 °C. • Products and reactants at these conditions are said to be in their standard states. Note that solutions must be 1 M. • The “not” symbol is used to indicate standard conditions, e. g. ΔH°rxn.

Heat of Combustion • The heat from the reaction that completely burns 1 mole of a substance: • C + O 2(g) CO 2(g) + 393. 5 k. J, H = -393. 5 Container of ethanol vapour mixed with air, undergoing rapid combustion


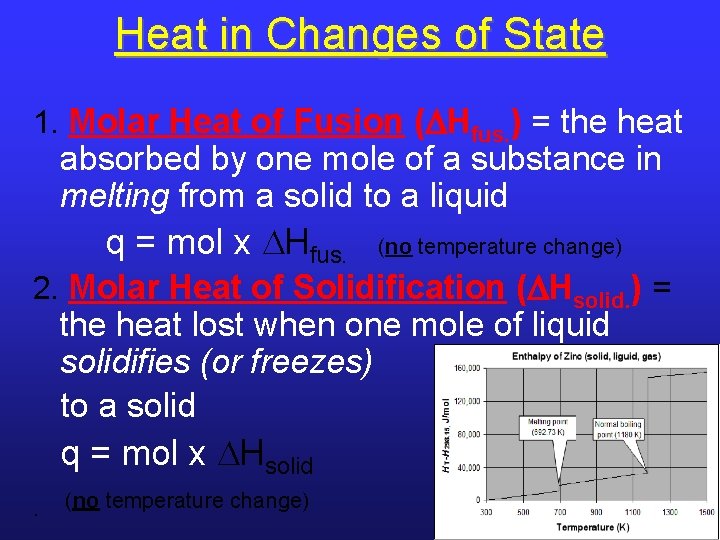
Heat in Changes of State 1. Molar Heat of Fusion ( Hfus. ) = the heat absorbed by one mole of a substance in melting from a solid to a liquid q = mol x Hfus. (no temperature change) 2. Molar Heat of Solidification ( Hsolid. ) = the heat lost when one mole of liquid solidifies (or freezes) to a solid q = mol x Hsolid. (no temperature change) 13

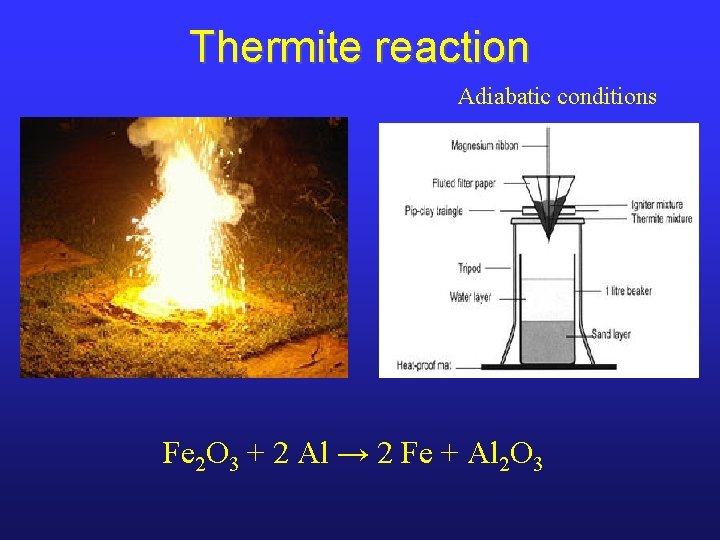
Thermite reaction Adiabatic conditions Fe 2 O 3 + 2 Al → 2 Fe + Al 2 O 3

Thermite reaction • Energy produced by the reaction itself should be calculate as: • subtracting the enthalpy of the reactants from the enthalpy of the products; • subtracting the energy consumed to heating the products (from their specific heat, when the materials only change their temperature), their enthalpy of fusion and eventually enthalpy of vaporization, when the materials melt or boil).

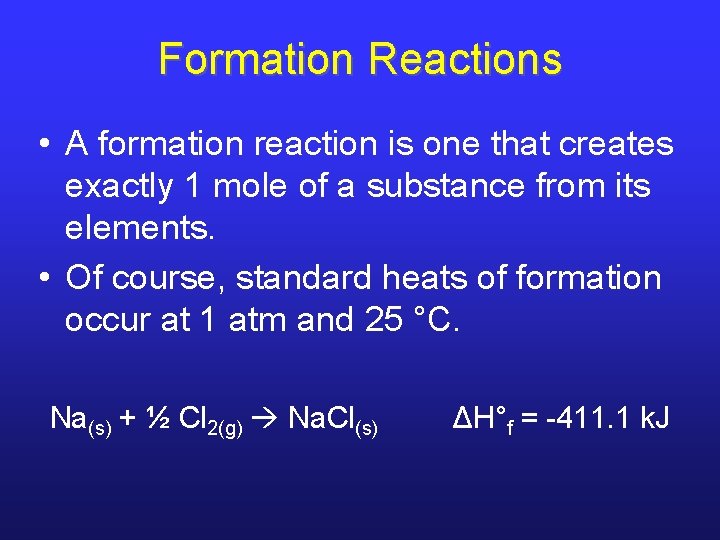
Formation Reactions • A formation reaction is one that creates exactly 1 mole of a substance from its elements. • Of course, standard heats of formation occur at 1 atm and 25 °C. Na(s) + ½ Cl 2(g) Na. Cl(s) ΔH°f = -411. 1 k. J

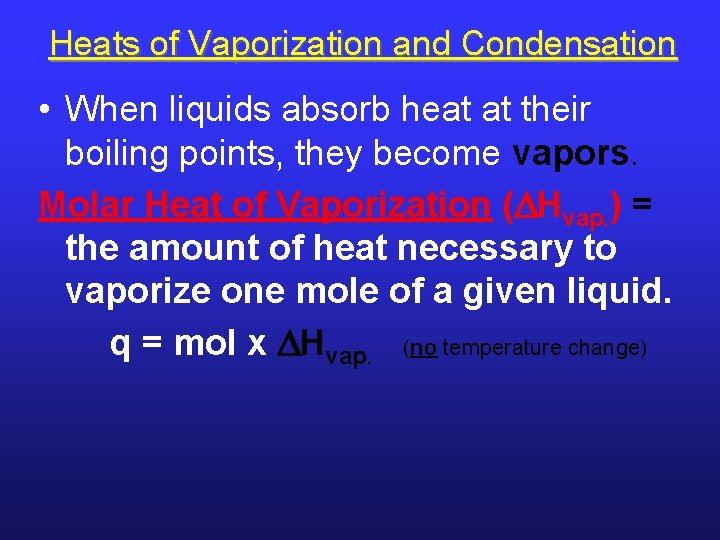
Heats of Vaporization and Condensation • When liquids absorb heat at their boiling points, they become vapors. Molar Heat of Vaporization ( Hvap. ) = the amount of heat necessary to vaporize one mole of a given liquid. q = mol x Hvap. (no temperature change)

Heats of Vaporization and Condensation • Condensation is the opposite of vaporization. Molar Heat of Condensation ( Hcond. ) = amount of heat released when one mole of vapor condenses to a liquid q = mol x Hcond. (no temperature change) • Hvap. = - Hcond.

Heats of Vaporization and Condensation • The large values for water Hvap. and Hcond. is the reason hot vapors such as steam are very dangerous! – You can receive a scalding burn from steam when the heat of condensation is released! H 20(g) H 20(l) Hcond. = - 40. 7 k. J/mol

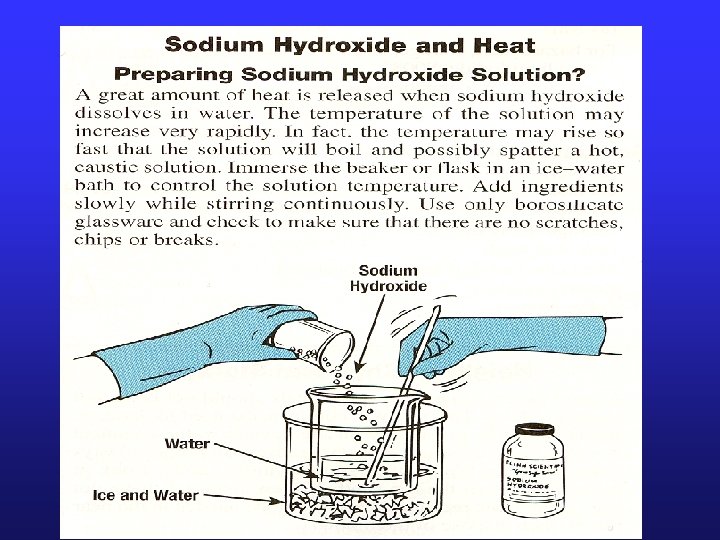
Heat of Solution • Heat changes can also occur when a solute dissolves in a solvent. Molar Heat of Solution ( Hsoln. ) = heat change caused by dissolution of one mole of substance; q = mol x Hsoln. • Sodium hydroxide provides a good example of an exothermic molar heat of solution (next slide) 20

Heat of Solution H 2 O(l) Na. OH(s) Na 1+(aq) + OH 1 -(aq) Hsoln. = - 445. 1 k. J/mol • The heat is released as the ions separate (by dissolving) and interact with water, releasing 445. 1 k. J of heat as Hsoln. – thus becoming so hot it steams! 21


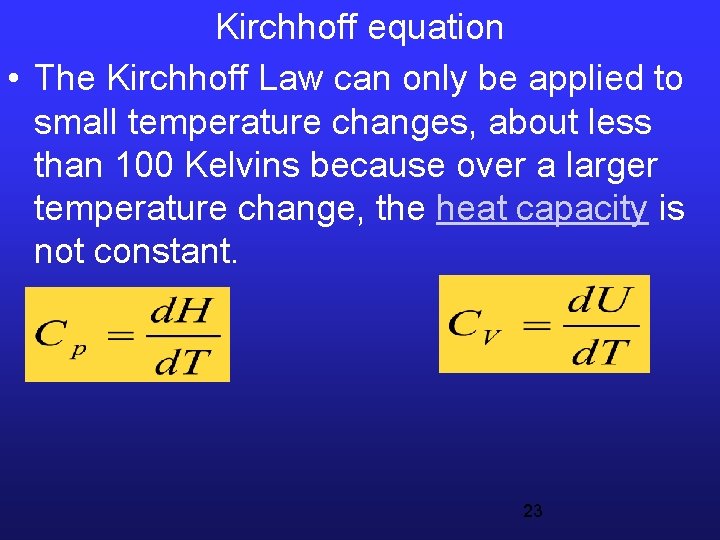
Kirchhoff equation • The Kirchhoff Law can only be applied to small temperature changes, about less than 100 Kelvins because over a larger temperature change, the heat capacity is not constant. 23

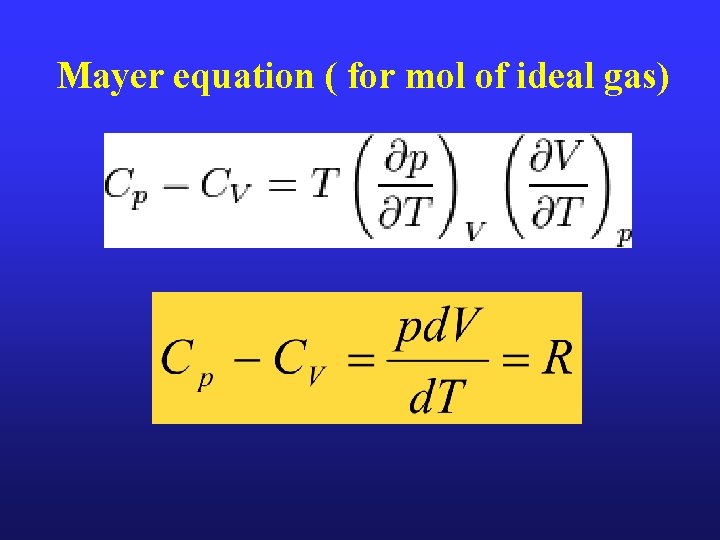
Mayer equation ( for mol of ideal gas)

Using ΔH°f’s • Standard heats of formation are very powerful; they allow calculation of ΔH°rxn for anything using a Hess’s Law type of calculation. ΔH°rxn = (sum ΔH°f products) – (sum ΔH°f reactants)


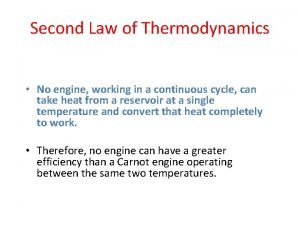
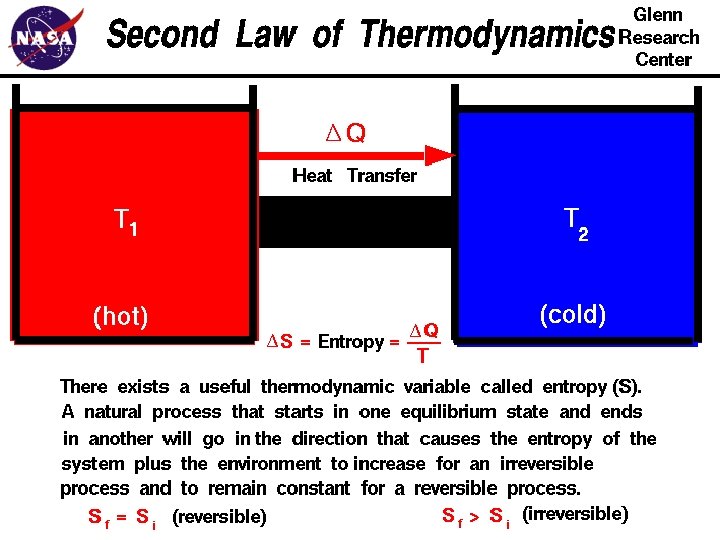
The second law of thermodynamics • states that the entropy of an isolated system never decreases, because isolated systems spontaneously evolve towards thermodynamic equilibrium—the state of maximum entropy.

Entropy Changes • In general, entropy increases when – Gases are formed from liquids and solids. – Liquids or solutions are formed from solids. – The number of gas molecules increases. – The number of moles increases.

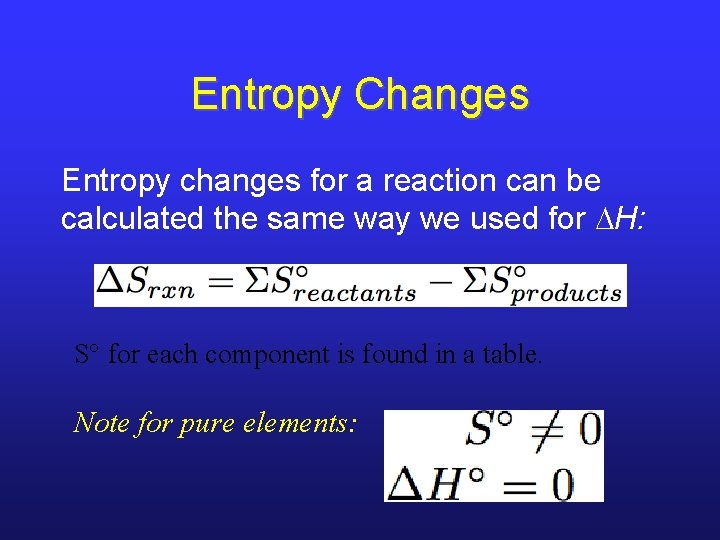
Entropy Changes Entropy changes for a reaction can be calculated the same way we used for H: S° for each component is found in a table. Note for pure elements:

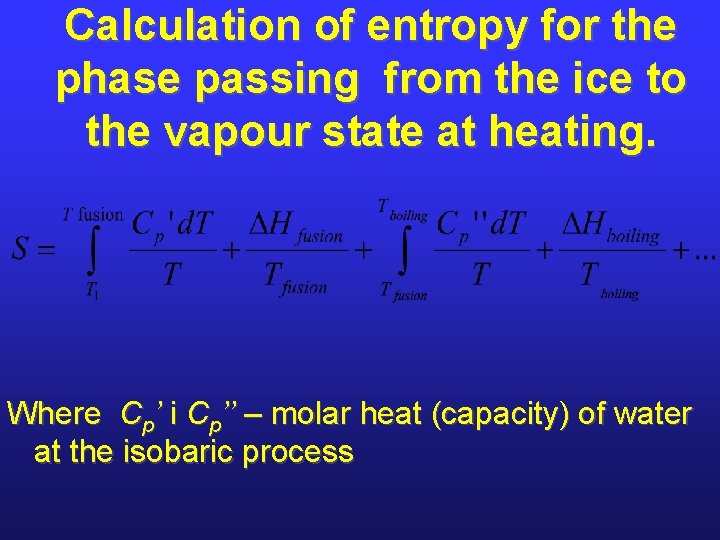
Calculation of entropy for the phase passing from the ice to the vapour state at heating. Where Ср’ і Ср’’ – molar heat (capacity) of water at the isobaric process
 Formula for entropy change
Formula for entropy change State second law of thermodynamics
State second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Second law of thermodynamics definition
Second law of thermodynamics definition Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law and second law and third law
Newton's first law and second law and third law Halina abramczyk
Halina abramczyk Halina abramowicz
Halina abramowicz Halina koczyk
Halina koczyk Bars halina kalemba
Bars halina kalemba Halina flisiak-antonijczuk
Halina flisiak-antonijczuk Halina baran
Halina baran Halina sitko
Halina sitko Kryzys oprogramowania
Kryzys oprogramowania Baran datum
Baran datum Kirchhoff's law enthalpy example
Kirchhoff's law enthalpy example 186 282 miles per second into meters per second
186 282 miles per second into meters per second Nozzle and diffuser
Nozzle and diffuser Zeroth law example
Zeroth law example Law of thermodynamics in chemistry
Law of thermodynamics in chemistry Zeroth law of thermodynamics statement
Zeroth law of thermodynamics statement Isobaric process formula
Isobaric process formula Cyclic process thermodynamics
Cyclic process thermodynamics Joules experiment
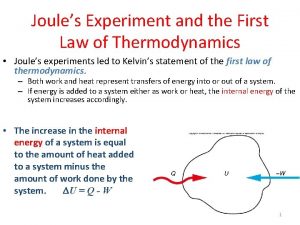
Joules experiment First law of thermodynamics
First law of thermodynamics In a flow process the work transfer may be of which type
In a flow process the work transfer may be of which type What are the laws of thermodynamics
What are the laws of thermodynamics Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law