Thermochemistry Part 2 SCH 4 U Heat and









- Slides: 27

Thermochemistry: Part 2 SCH 4 U

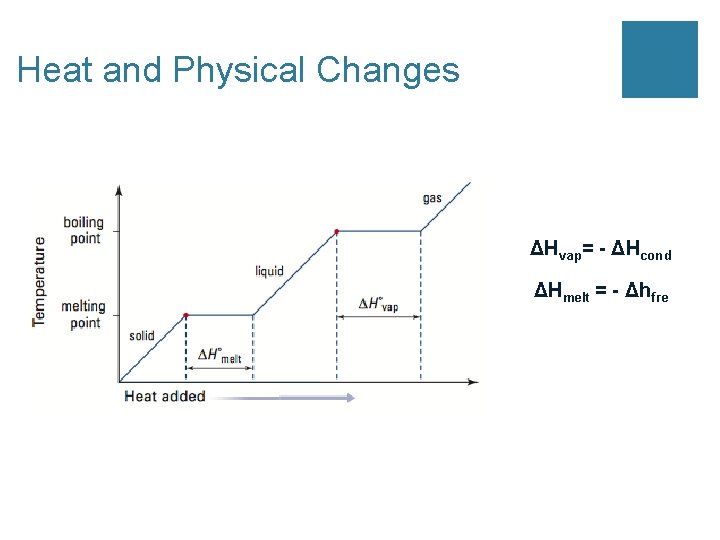
Heat and Physical Changes ΔHvap= - ΔHcond ΔHmelt = - Δhfre

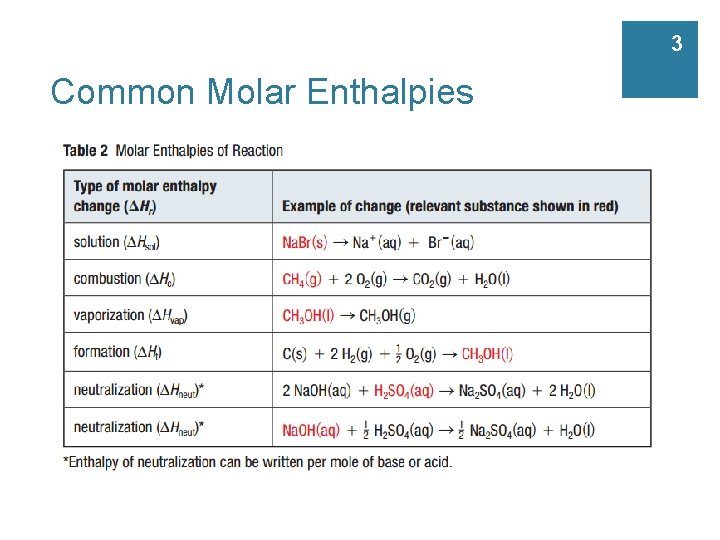
3 Common Molar Enthalpies

Methods of Measuring ΔH 1. Calorimetry (experimental) 2. Hess’s Law: using Standard Enthalpy of Reaction (ΔHrxn) of a series of reaction steps (indirect method). 3. Standard Enthalpy of Formation (ΔHf ) used with Hess’s Law (direct method) 4. Bond Energies used with Hess’s Law

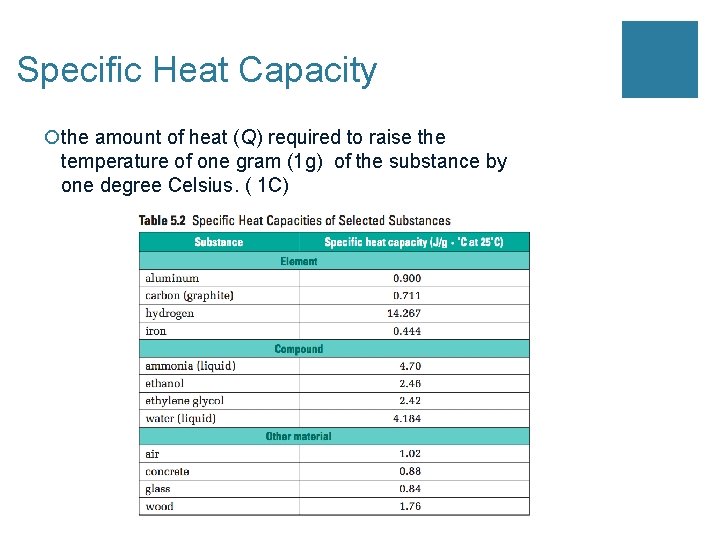
Specific Heat Capacity ¡the amount of heat (Q) required to raise the temperature of one gram (1 g) of the substance by one degree Celsius. ( 1 C)

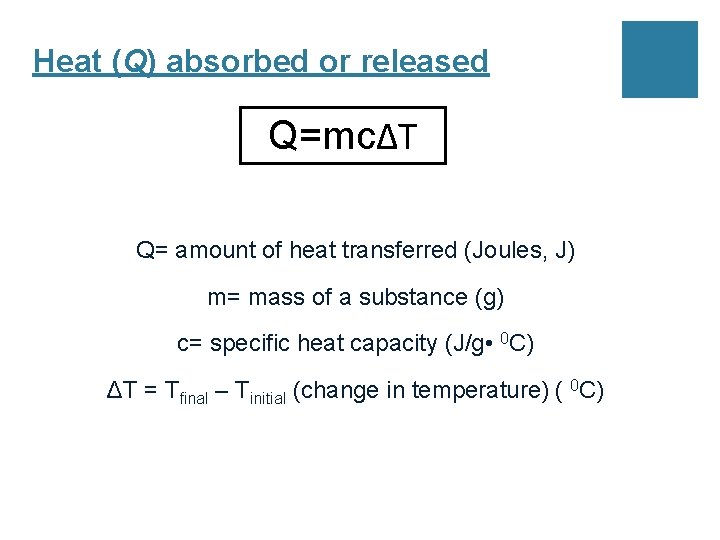
Heat (Q) absorbed or released Q=mcΔT Q= amount of heat transferred (Joules, J) m= mass of a substance (g) c= specific heat capacity (J/g • 0 C) ΔT = Tfinal – Tinitial (change in temperature) ( 0 C)

Heat Examples 1) When a 1. 25 kg sample of water was heated in a kettle, its temperature increased from 16. 40 C to 98. 90 C. How much heat did the water absorb?

Heat Examples 1) When a 1. 25 kg sample of water was heated in a kettle, its temperature increased from 16. 40 C to 98. 90 C. How much heat did the water absorb? Q= 1. 25 x 103 g x 4. 18 J/g 0 C x (+82. 50 C ) = 432 093. 75 J = 4. 32 x 105 J

Heat Examples 2) How much heat must be added to 128. 62 g of steam at 126. 00 C to increase its temperature to 189. 50 C? 3) A solid substance has a mass of 250. 0 g. It is cooled by 25. 000 C and loses 4. 937 k. J of heat. What is the specific heat capacity of the substance?

Heat Examples 2) How much heat must be added to 128. 62 g of steam at 126. 00 C to increase its temperature to 189. 50 C? Q = 128. 62 gx 2. 02 J/g 0 Cx (189. 5 -126. 0) 0 C = 1. 65 x 104 J 3) A solid substance has a mass of 250. 0 g. It is cooled by 25. 000 C and loses 4. 937 k. J of heat. What is the specific heat capacity of the substance? c= Q/m(∆T) c= -4. 937 x 103 J/(250. 0 g (-25. 000 C)) c= 0. 7899 J/g 0 C

Additional Examples 1) How much heat is released when the temperature of 789 g of liquid ammonia decreases from 82. 70 C to 25. 00 C? 2) On a warm day, how much solar energy does a 3. 982 kg piece of concrete absorb as heat if its temperature increases from 13. 600 C to 14. 500 C

Additional Examples 1) How much heat is released when the temperature of 789 g of liquid ammonia decreases from 82. 70 C to 25. 00 C? Q= mc∆T = 789 g x 4. 70 J/g 0 C x (25. 0 -82. 7) = - 213968. 91 J change to -2. 14 x 105 J 2) On a warm day, how much solar energy does a 3. 982 kg piece of concrete absorb as heat if its temperature increases from 13. 600 C to 14. 500 C? Q= 3982 g x 0. 88 J/g 0 C x (14. 50 -13. 60) = 3153. 744 J change to 3. 2 x 103 J

Measuring Q experimentally ¡Calorimeter a device that is used to measure enthalpy changes for chemical and physical reactions. Qreactions = - Q insulated system

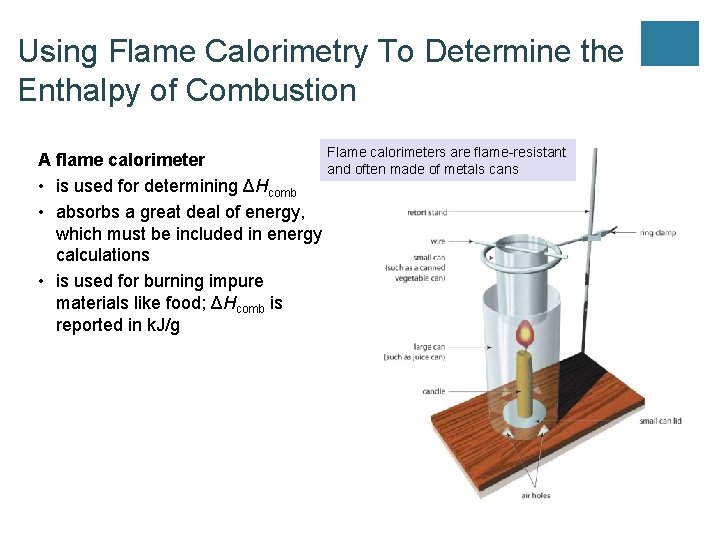
UNIT 3 Chapter 5: Energy Changes Section 5. 2 Using Flame Calorimetry To Determine the Enthalpy of Combustion A flame calorimeter • is used for determining ΔHcomb • absorbs a great deal of energy, which must be included in energy calculations • is used for burning impure materials like food; ΔHcomb is reported in k. J/g Flame calorimeters are flame-resistant and often made of metals cans

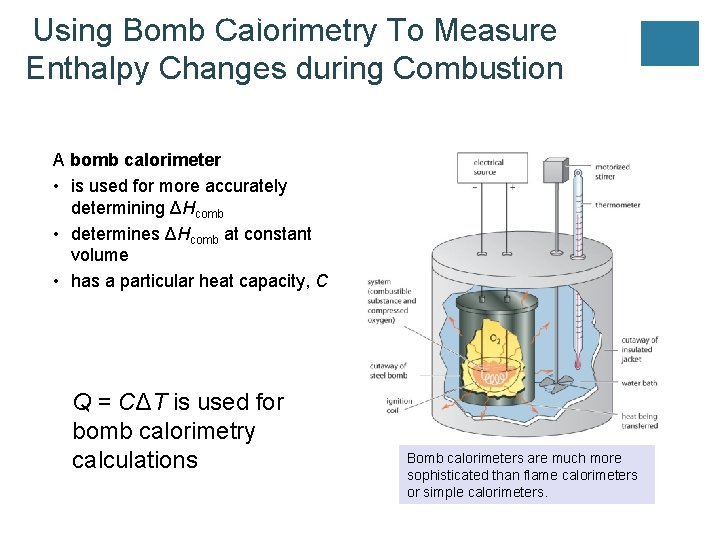
UNIT 3 Chapter 5: Energy Changes Using Bomb Calorimetry To Measure Enthalpy Changes during Combustion Section 5. 2 A bomb calorimeter • is used for more accurately determining ΔHcomb • determines ΔHcomb at constant volume • has a particular heat capacity, C Q = CΔT is used for bomb calorimetry calculations Bomb calorimeters are much more sophisticated than flame calorimeters or simple calorimeters.

UNIT 3 Chapter 5: Energy Changes Section 5. 2 Using a Simple Calorimeter • Constant-Pressure Calorimeter • No boundary between system and surroundings • Reactants and products are system, and water in which they dissolve in, is part of the surroundings • the change in temperature of the water is measured; the solution absorbs or releases energy • the solution is dilute enough so that the specific heat capacity of water is used Thermometer Styrofoam cover Styrofoam cups Stirrer

Practice #1

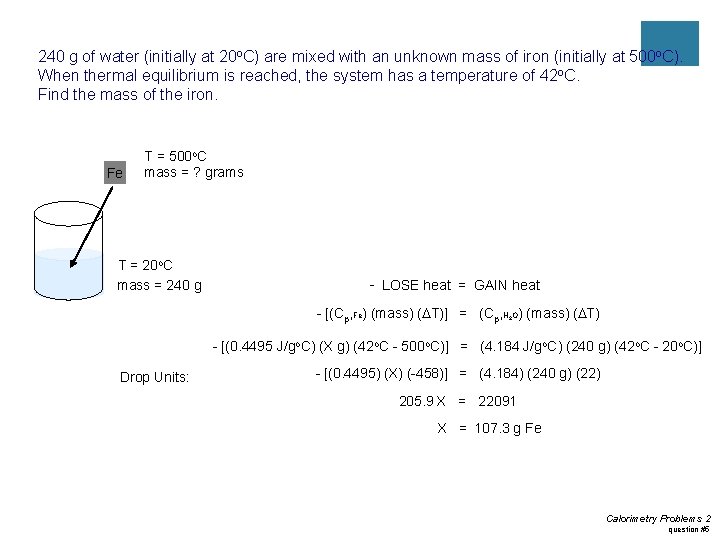
Practice #2 ¡ 240 g of water (initially at 20 o. C) are mixed with an unknown mass of iron (initially at 500 o. C). When thermal equilibrium is reached, the system has a temperature of 42 o. C. Find the mass of the iron.

240 g of water (initially at 20 o. C) are mixed with an unknown mass of iron (initially at 500 o. C). When thermal equilibrium is reached, the system has a temperature of 42 o. C. Find the mass of the iron. Fe T = 500 o. C mass = ? grams T = 20 o. C mass = 240 g - LOSE heat = GAIN heat - [(Cp, Fe) (mass) (DT)] = (Cp, H O) (mass) (DT) 2 - [(0. 4495 J/go. C) (X g) (42 o. C - 500 o. C)] = (4. 184 J/go. C) (240 g) (42 o. C - 20 o. C)] Drop Units: - [(0. 4495) (X) (-458)] = (4. 184) (240 g) (22) 205. 9 X = 22091 X = 107. 3 g Fe Calorimetry Problems 2 question #5

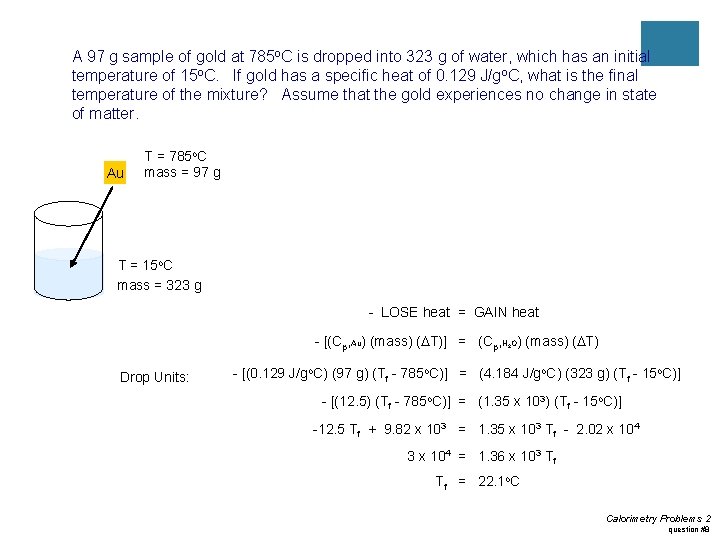
Practice #3 ¡A 97 g sample of gold at 785 o. C is dropped into 323 g of water, which has an initial temperature of 15 o. C. If gold has a specific heat of 0. 129 J/go. C, what is the final temperature of the mixture? Assume that the gold experiences no change in state of matter.

A 97 g sample of gold at 785 o. C is dropped into 323 g of water, which has an initial temperature of 15 o. C. If gold has a specific heat of 0. 129 J/go. C, what is the final temperature of the mixture? Assume that the gold experiences no change in state of matter. Au T = 785 o. C mass = 97 g T = 15 o. C mass = 323 g - LOSE heat = GAIN heat - [(Cp, Au) (mass) (DT)] = (Cp, H O) (mass) (DT) 2 Drop Units: - [(0. 129 J/go. C) (97 g) (Tf - 785 o. C)] = (4. 184 J/go. C) (323 g) (Tf - 15 o. C)] - [(12. 5) (Tf - 785 o. C)] = (1. 35 x 103) (Tf - 15 o. C)] -12. 5 Tf + 9. 82 x 103 = 1. 35 x 103 Tf - 2. 02 x 104 3 x 104 = 1. 36 x 103 Tf Tf = 22. 1 o. C Calorimetry Problems 2 question #8

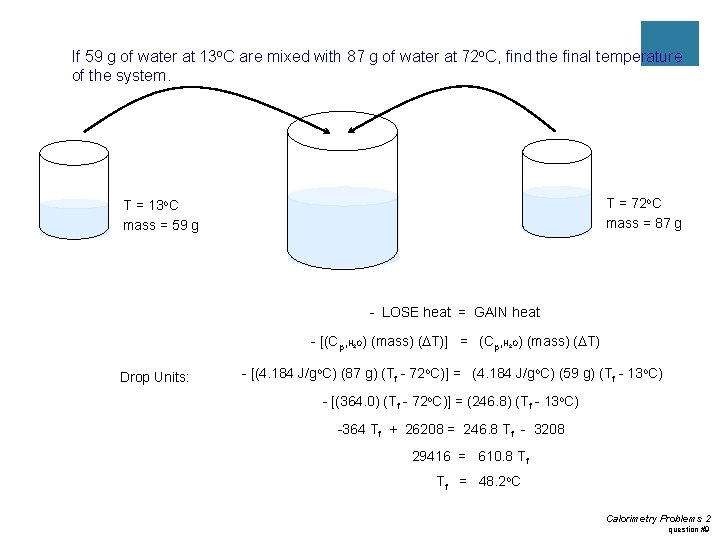
Practice #4 ¡If 59 g of water at 13 o. C are mixed with 87 g of water at 72 o. C, find the final temperature of the system.

If 59 g of water at 13 o. C are mixed with 87 g of water at 72 o. C, find the final temperature of the system. T = 72 o. C mass = 87 g T = 13 o. C mass = 59 g - LOSE heat = GAIN heat - [(Cp, H O) (mass) (DT)] = (Cp, H O) (mass) (DT) 2 Drop Units: 2 - [(4. 184 J/go. C) (87 g) (Tf - 72 o. C)] = (4. 184 J/go. C) (59 g) (Tf - 13 o. C) - [(364. 0) (Tf - 72 o. C)] = (246. 8) (Tf - 13 o. C) -364 Tf + 26208 = 246. 8 Tf - 3208 29416 = 610. 8 Tf Tf = 48. 2 o. C Calorimetry Problems 2 question #9

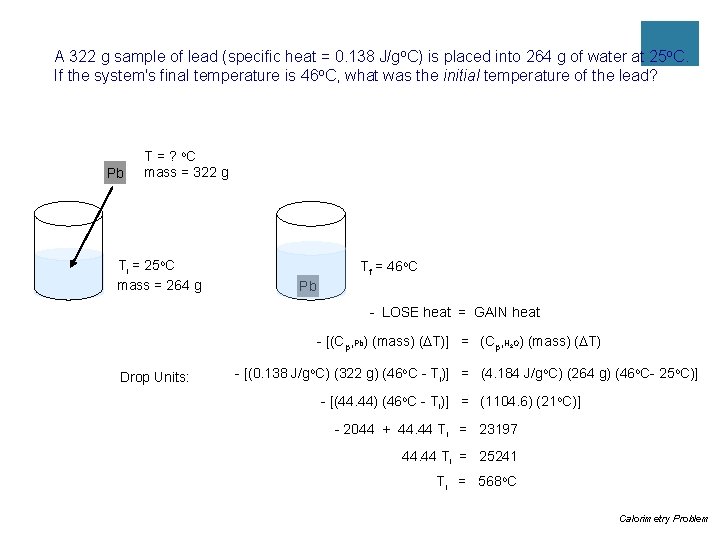
Practice #5 ¡A 322 g sample of lead (specific heat = 0. 138 J/go. C) is placed into 264 g of water at 25 o. C. If the system's final temperature is 46 o. C, what was the initial temperature of the lead?

A 322 g sample of lead (specific heat = 0. 138 J/go. C) is placed into 264 g of water at 25 o. C. If the system's final temperature is 46 o. C, what was the initial temperature of the lead? Pb T = ? o. C mass = 322 g Ti = 25 o. C mass = 264 g Tf = 46 o. C Pb - LOSE heat = GAIN heat - [(Cp, Pb) (mass) (DT)] = (Cp, H O) (mass) (DT) 2 Drop Units: - [(0. 138 J/go. C) (322 g) (46 o. C - Ti)] = (4. 184 J/go. C) (264 g) (46 o. C- 25 o. C)] - [(44. 44) (46 o. C - Ti)] = (1104. 6) (21 o. C)] - 2044 + 44. 44 Ti = 23197 44. 44 Ti = 25241 Ti = 568 o. C Calorimetry Problem

26 Molar Enthalpy Change ΔHn: ¡Molar Enthalpy Change (ΔHn): enthalpy change associated with any change (physical, chemical or nuclear) in one mole of a substance; J/mol or k. J/mol ΔHrxn = nΔHn

27 Example: ¡Ethanol is used to disinfect skin before receiving a flu shot. How does this feel on your skin? The enthalpy of vaporization (ΔHvap)of ethanol is 38. 6 k. J/mol. What is the enthalpy change (ΔHrxn) of this reaction if 1. 0 g of ethanol is rubbed on your skin?