Thermochemistry Energy of Chemical Reactions ORThe study of




- Slides: 28

Thermochemistry -Energy of Chemical Reactions OR-The study of heat changes that occur during chemical reactions and physical changes of state 1

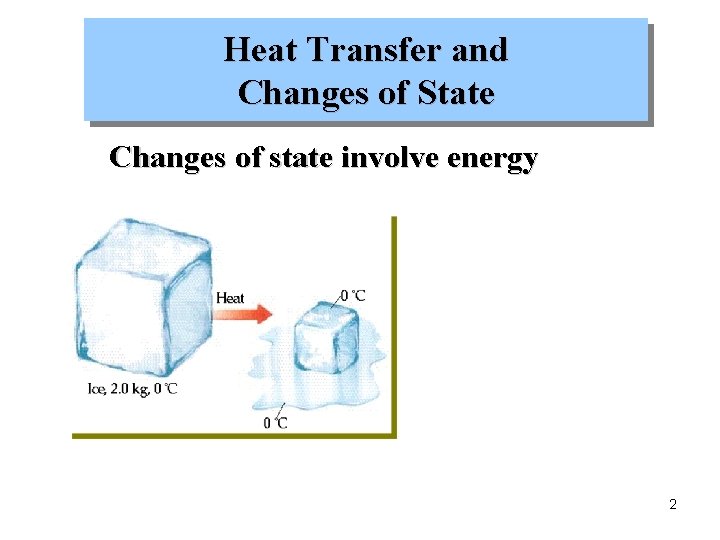
Heat Transfer and Changes of State Changes of state involve energy 2

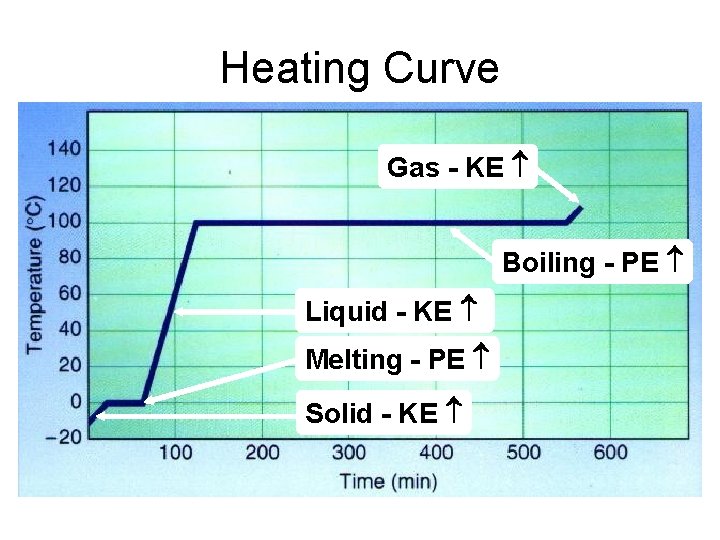
Heating Curve Gas - KE Boiling - PE Liquid - KE Melting - PE Solid - KE

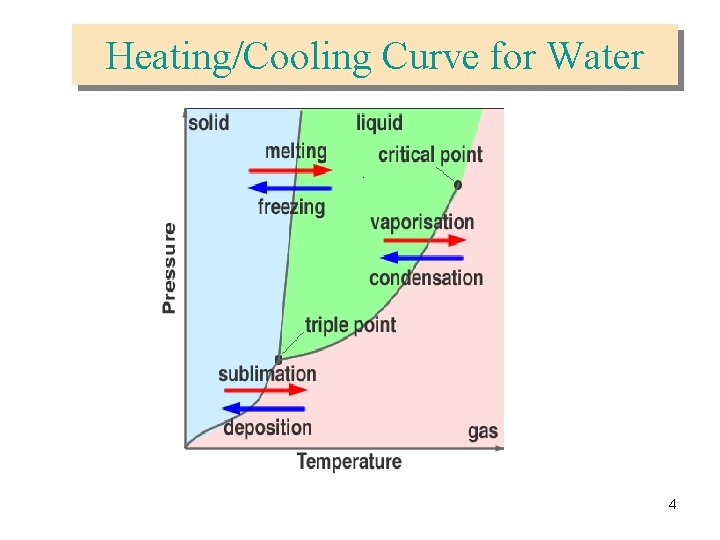
Heating/Cooling Curve for Water 4

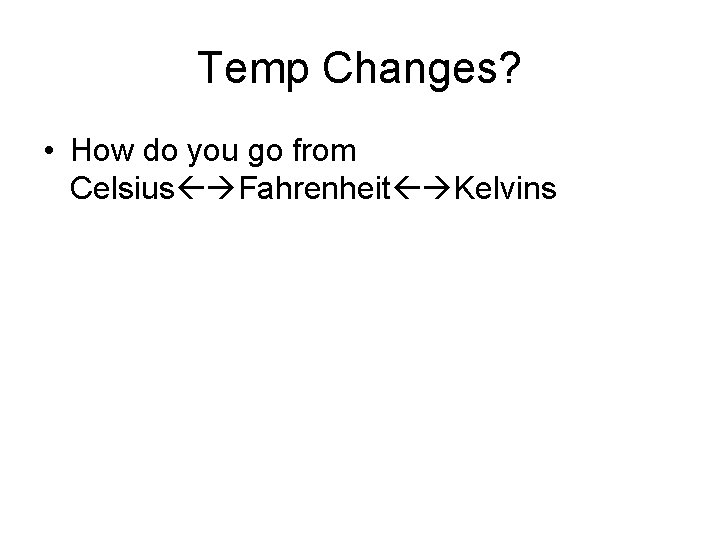
Temp Changes? • How do you go from Celsius Fahrenheit Kelvins

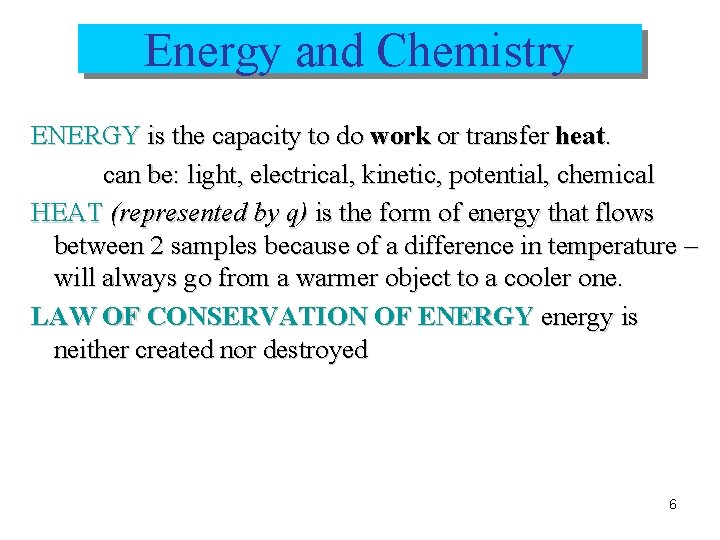
Energy and Chemistry ENERGY is the capacity to do work or transfer heat. can be: light, electrical, kinetic, potential, chemical HEAT (represented by q) is the form of energy that flows between 2 samples because of a difference in temperature – will always go from a warmer object to a cooler one. LAW OF CONSERVATION OF ENERGY energy is neither created nor destroyed 6

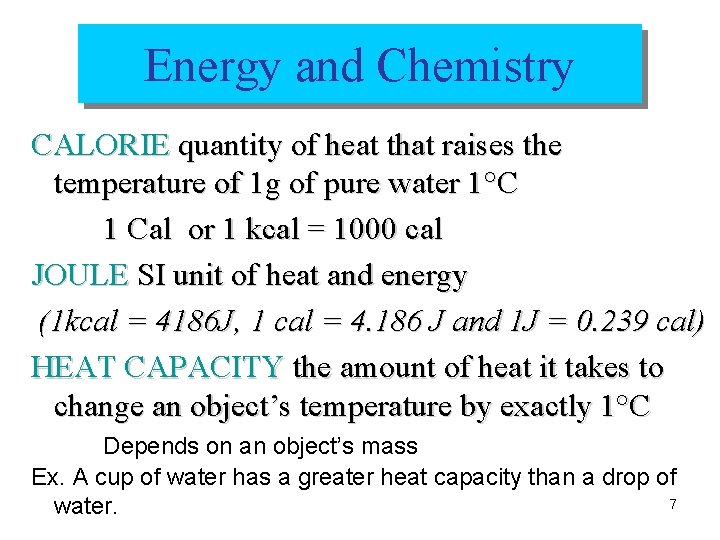
Energy and Chemistry CALORIE quantity of heat that raises the temperature of 1 g of pure water 1°C 1 Cal or 1 kcal = 1000 cal JOULE SI unit of heat and energy (1 kcal = 4186 J, 1 cal = 4. 186 J and 1 J = 0. 239 cal) HEAT CAPACITY the amount of heat it takes to change an object’s temperature by exactly 1°C Depends on an object’s mass Ex. A cup of water has a greater heat capacity than a drop of 7 water.

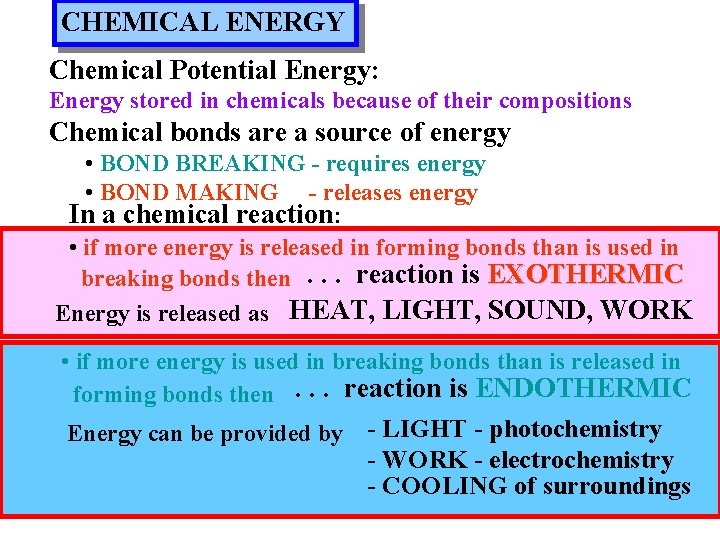
CHEMICAL ENERGY Chemical Potential Energy: Energy stored in chemicals because of their compositions Chemical bonds are a source of energy • BOND BREAKING - requires energy • BOND MAKING - releases energy In a chemical reaction: • if more energy is released in forming bonds than is used in breaking bonds then. . . reaction is EXOTHERMIC Energy is released as HEAT, LIGHT, SOUND, WORK • if more energy is used in breaking bonds than is released in forming bonds then. . . reaction is ENDOTHERMIC Energy can be provided by - LIGHT - photochemistry - WORK - electrochemistry - COOLING of surroundings

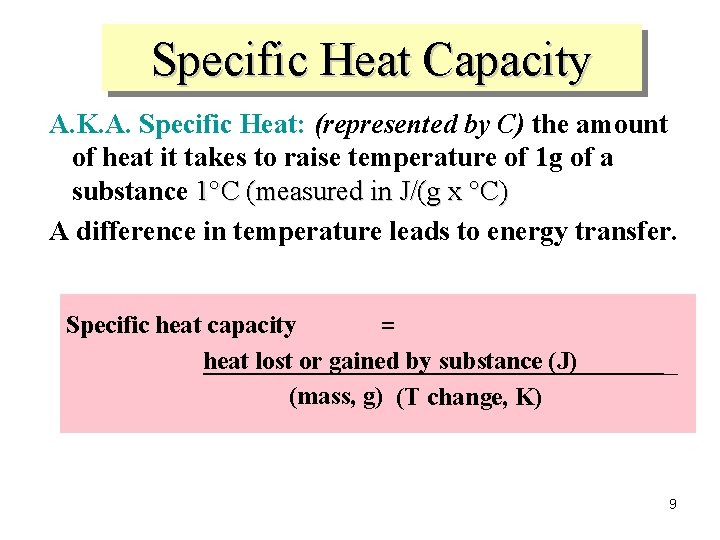
Specific Heat Capacity A. K. A. Specific Heat: (represented by C) the amount of heat it takes to raise temperature of 1 g of a substance 1°C (measured in J/(g x °C) A difference in temperature leads to energy transfer. Specific heat capacity = heat lost or gained by substance (J) (mass, g) (T change, K) 9

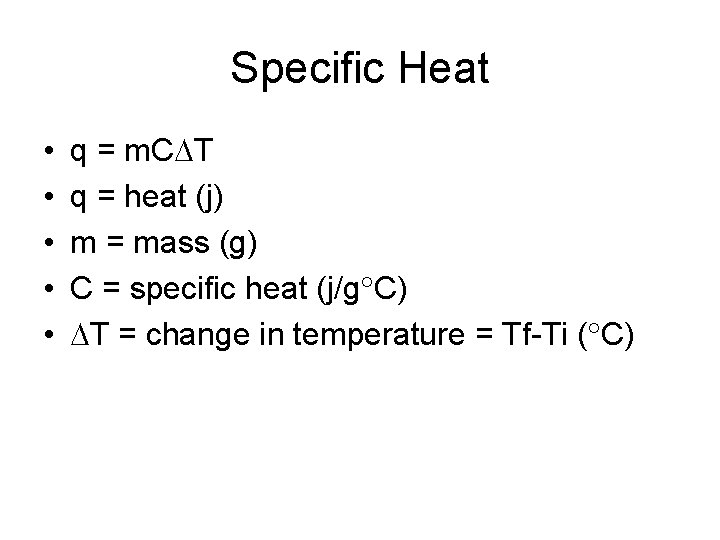
Specific Heat • • • q = m. C T q = heat (j) m = mass (g) C = specific heat (j/g C) T = change in temperature = Tf-Ti ( C)

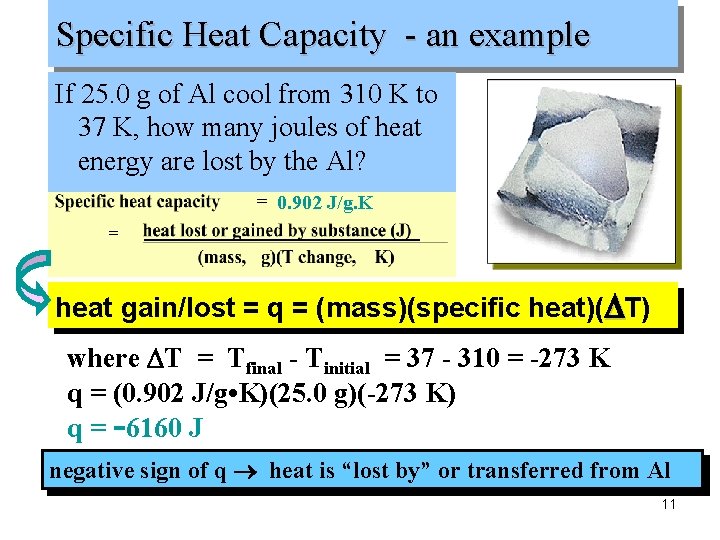
Specific Heat Capacity - an example If 25. 0 g of Al cool from 310 K to 37 K, how many joules of heat energy are lost by the Al? 0. 902 J/g. K = heat gain/lost = q = (mass)(specific heat)(DT) where DT = Tfinal - Tinitial = 37 - 310 = -273 K q = (0. 902 J/g • K)(25. 0 g)(-273 K) q = -6160 J negative sign of q heat is “lost by” or transferred from Al 11

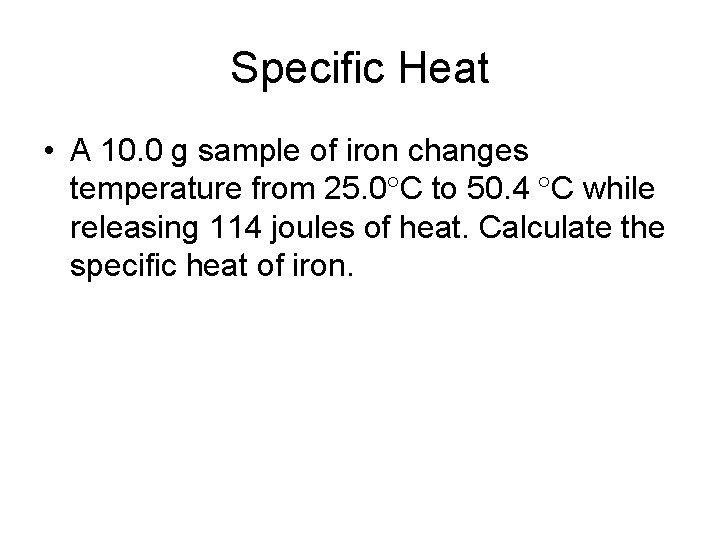
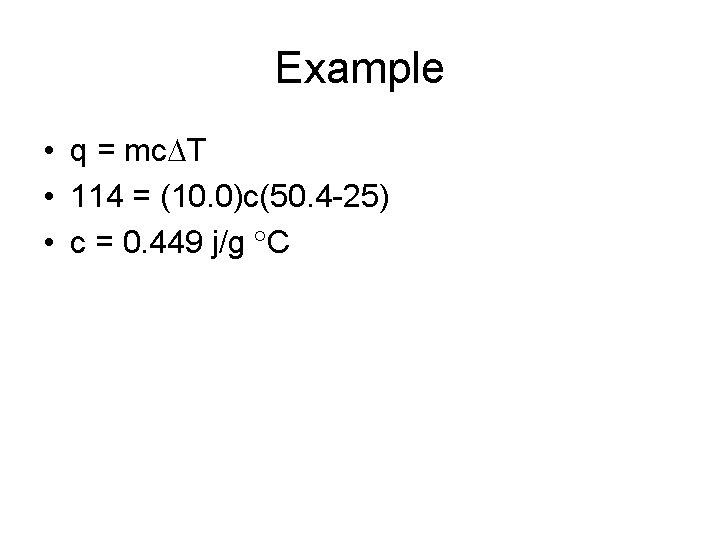
Specific Heat • A 10. 0 g sample of iron changes temperature from 25. 0 C to 50. 4 C while releasing 114 joules of heat. Calculate the specific heat of iron.

Example • q = mc T • 114 = (10. 0)c(50. 4 -25) • c = 0. 449 j/g C

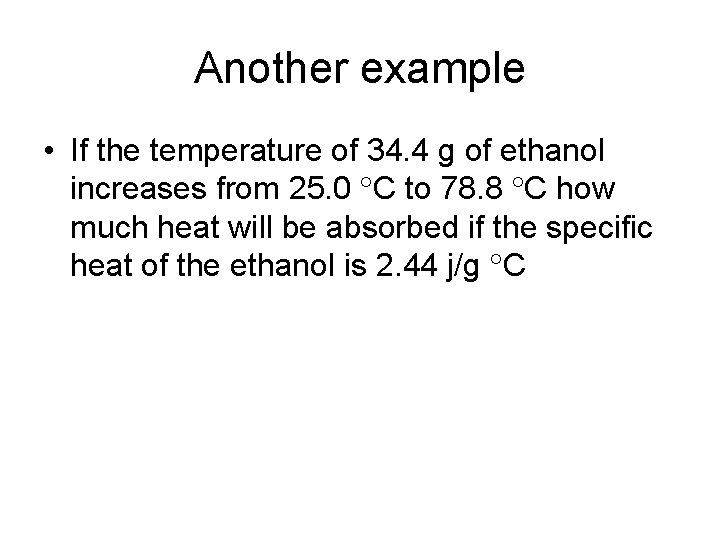
Another example • If the temperature of 34. 4 g of ethanol increases from 25. 0 C to 78. 8 C how much heat will be absorbed if the specific heat of the ethanol is 2. 44 j/g C

Another example • q = mc T • q = (34. 4)(2. 44)(78. 8 -25) • q = 4. 53 x 10 3 j

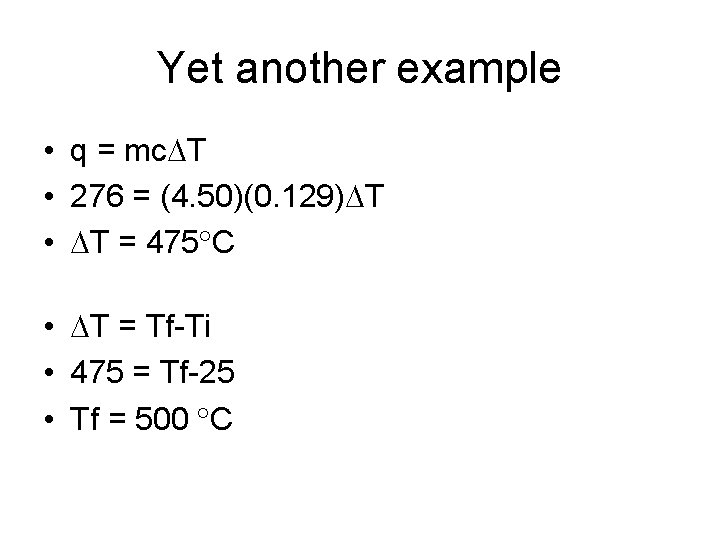
Yet another example • 4. 50 g of a gold nugget absorbs 276 J of heat. What is the final temperature of the gold if the initial temperature was 25. 0 C & the specific heat of the gold is 0. 129 j/g C

Yet another example • q = mc T • 276 = (4. 50)(0. 129) T • T = 475 C • T = Tf-Ti • 475 = Tf-25 • Tf = 500 C

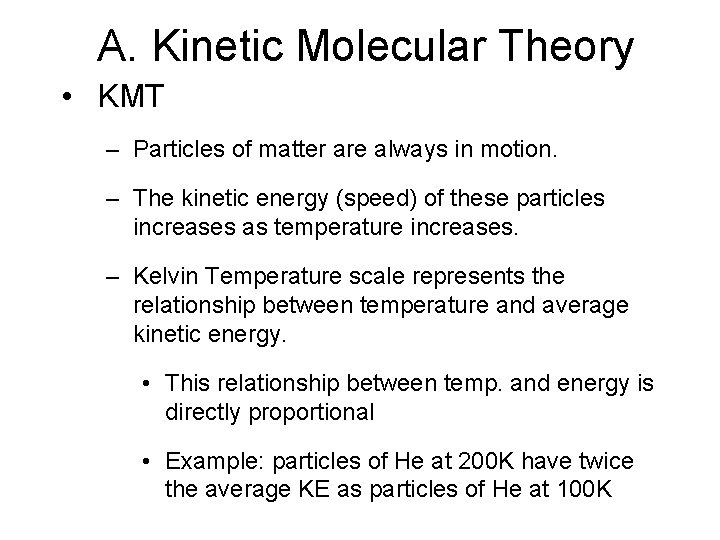
A. Kinetic Molecular Theory • KMT – Particles of matter are always in motion. – The kinetic energy (speed) of these particles increases as temperature increases. – Kelvin Temperature scale represents the relationship between temperature and average kinetic energy. • This relationship between temp. and energy is directly proportional • Example: particles of He at 200 K have twice the average KE as particles of He at 100 K

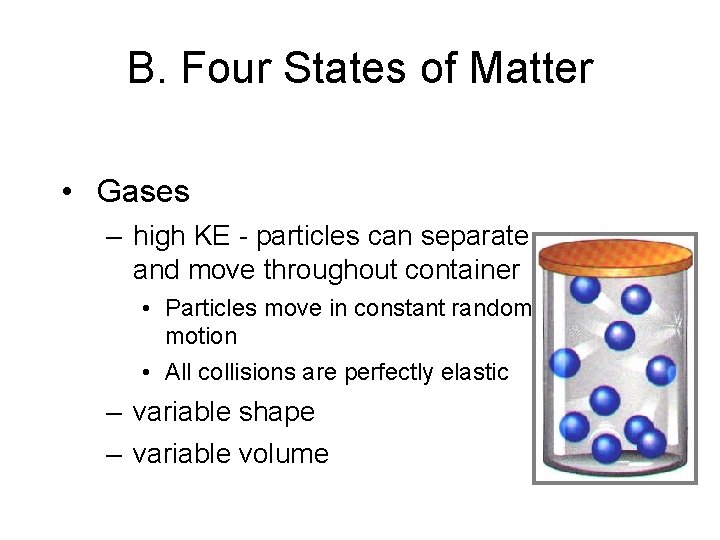
B. Four States of Matter • Gases – high KE - particles can separate and move throughout container • Particles move in constant random motion • All collisions are perfectly elastic – variable shape – variable volume

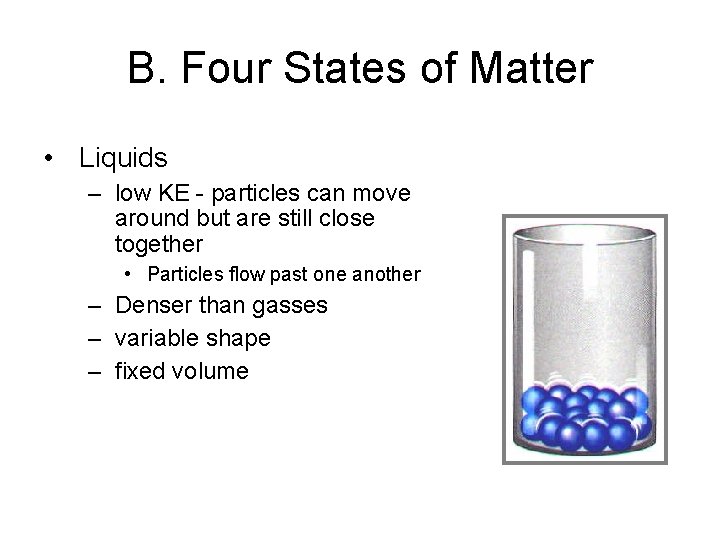
B. Four States of Matter • Liquids – low KE - particles can move around but are still close together • Particles flow past one another – Denser than gasses – variable shape – fixed volume

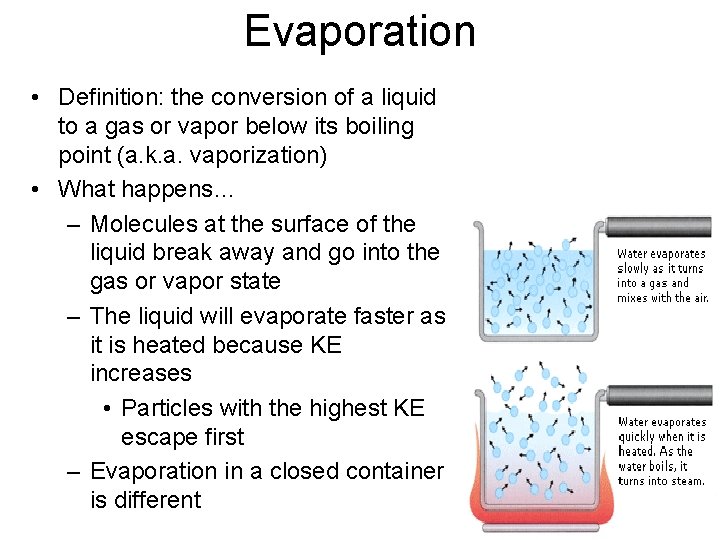
Evaporation • Definition: the conversion of a liquid to a gas or vapor below its boiling point (a. k. a. vaporization) • What happens… – Molecules at the surface of the liquid break away and go into the gas or vapor state – The liquid will evaporate faster as it is heated because KE increases • Particles with the highest KE escape first – Evaporation in a closed container is different

• Particles collide with the walls of the sealed container and produce a vapor pressure above the liquid. • As the container stands, the number of particles turning into vapor increases • Some of the particles will condense and turn to liquid • After some time vapor particles condensing will equal particles vaporizing to create an equilibrium

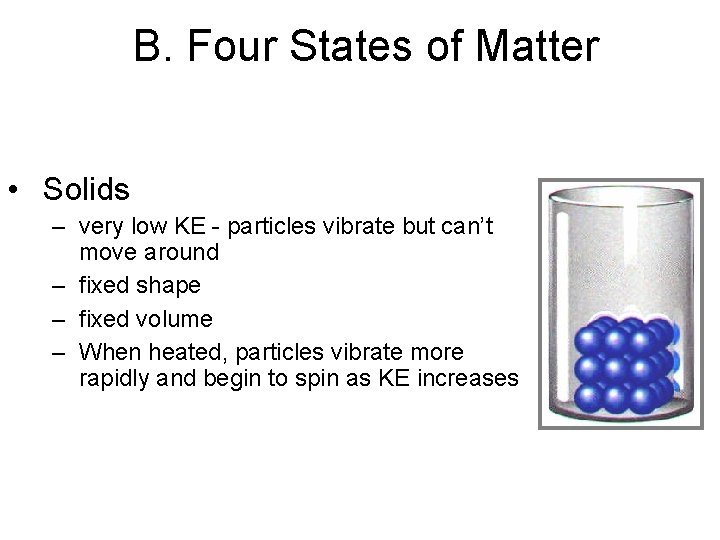
B. Four States of Matter • Solids – very low KE - particles vibrate but can’t move around – fixed shape – fixed volume – When heated, particles vibrate more rapidly and begin to spin as KE increases

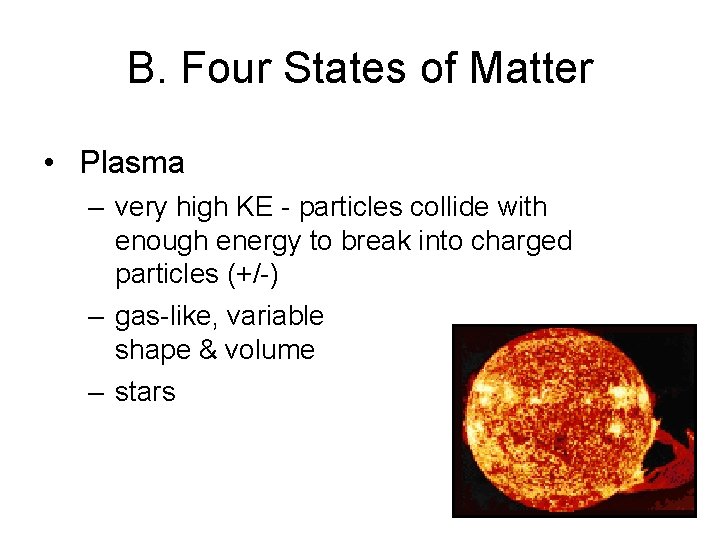
B. Four States of Matter • Plasma – very high KE - particles collide with enough energy to break into charged particles (+/-) – gas-like, variable shape & volume – stars

ENTHALPY For systems at constant pressure, the heat content is the same as the Enthalpy, or H, of the system. Heat changes for reactions carried out at constant pressure are the same as changes in enthalpy (∆H) DH = Hfinal - Hinitial If Hfinal > Hinitial then DH is positive Process is ENDOTHERMIC If Hfinal < Hinitial then DH is negative Process is EXOTHERMIC 25

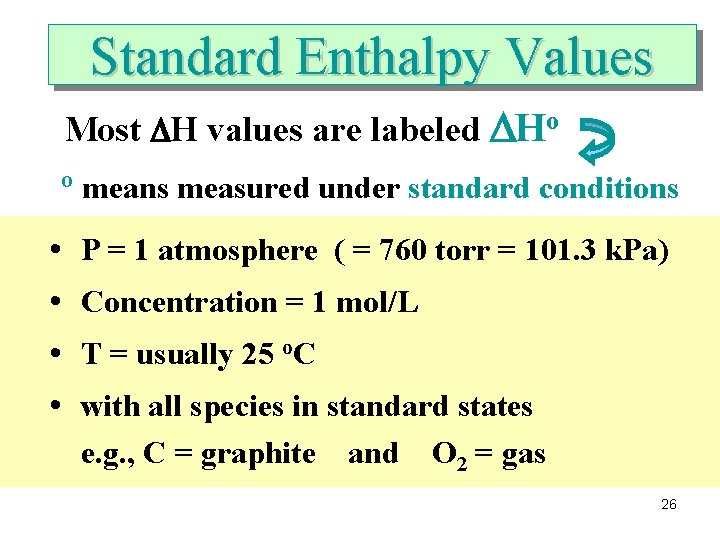
Standard Enthalpy Values Most DH values are labeled DHo o • • means measured under standard conditions P = 1 atmosphere ( = 760 torr = 101. 3 k. Pa) Concentration = 1 mol/L T = usually 25 o. C with all species in standard states e. g. , C = graphite and O 2 = gas 26

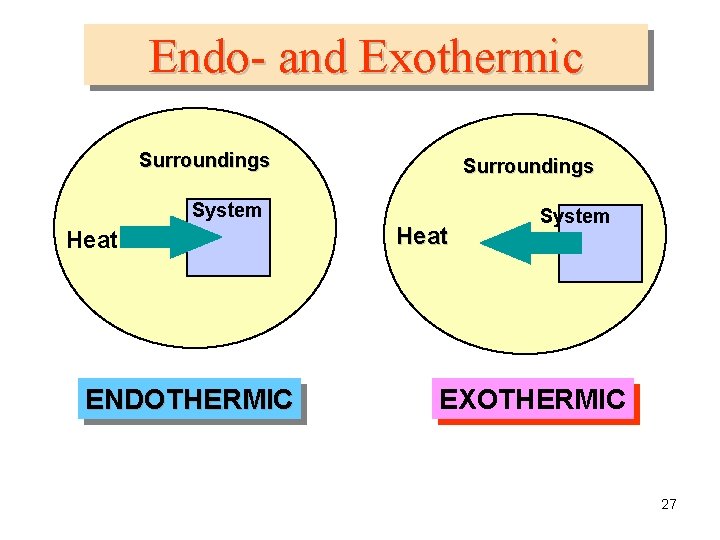
Endo- and Exothermic Surroundings System Heat ENDOTHERMIC Heat System EXOTHERMIC 27

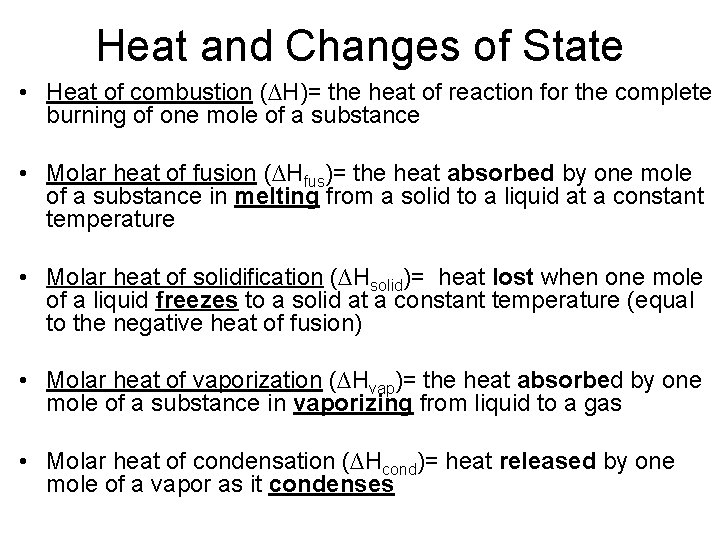
Heat and Changes of State • Heat of combustion (∆H)= the heat of reaction for the complete burning of one mole of a substance • Molar heat of fusion (∆Hfus)= the heat absorbed by one mole of a substance in melting from a solid to a liquid at a constant temperature • Molar heat of solidification (∆Hsolid)= heat lost when one mole of a liquid freezes to a solid at a constant temperature (equal to the negative heat of fusion) • Molar heat of vaporization (∆Hvap)= the heat absorbed by one mole of a substance in vaporizing from liquid to a gas • Molar heat of condensation (∆Hcond)= heat released by one mole of a vapor as it condenses