Thermochemistry Define Heat Compare Endothermic and Exothermic reactions





- Slides: 17

Thermochemistry Define Heat Compare Endothermic and Exothermic reactions Know units

Energy Transformations • Thermochemistry – the study of energy changes that occur during chemical reactions and changes in state • Chemical potential energy – energy stored in the chemical bonds of a substance

Heat • Represented by q • One effect of adding heat to an object is an increase in its temperature • Heat always flows from a warmer object to a cooler object

Processes • System – the part of the universe on which you focus your attention • Surroundings – everything else in the universe • Law of Conservation of Energy – in any chemical and physical process, energy is neither created or destroyed

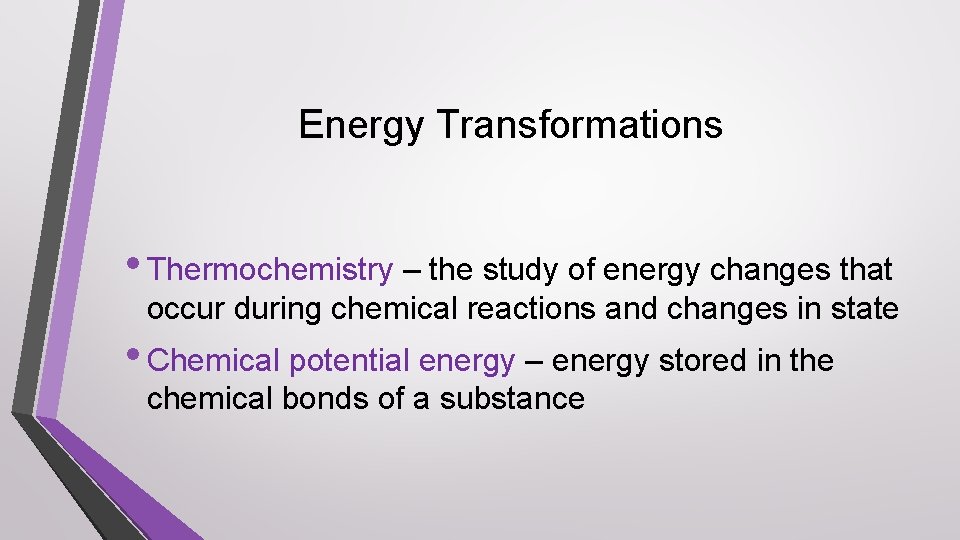
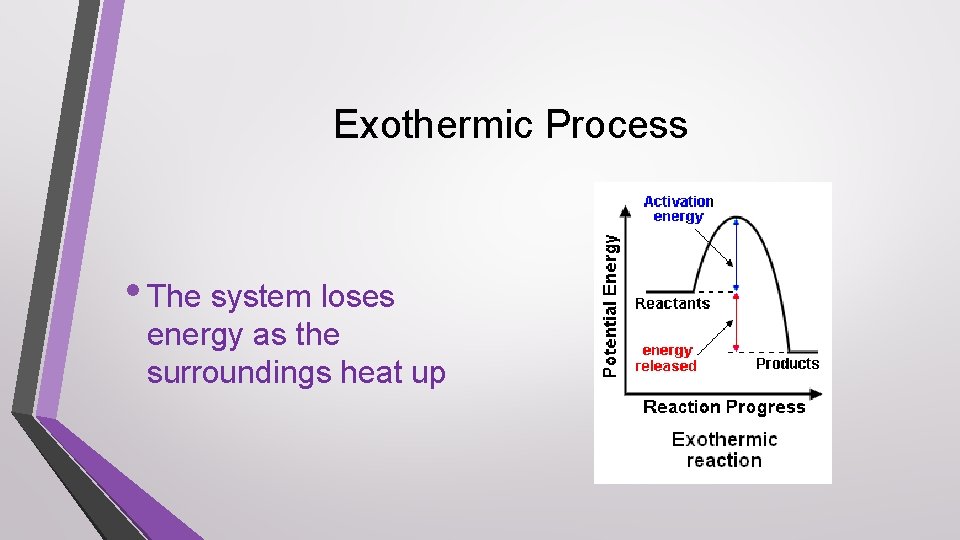
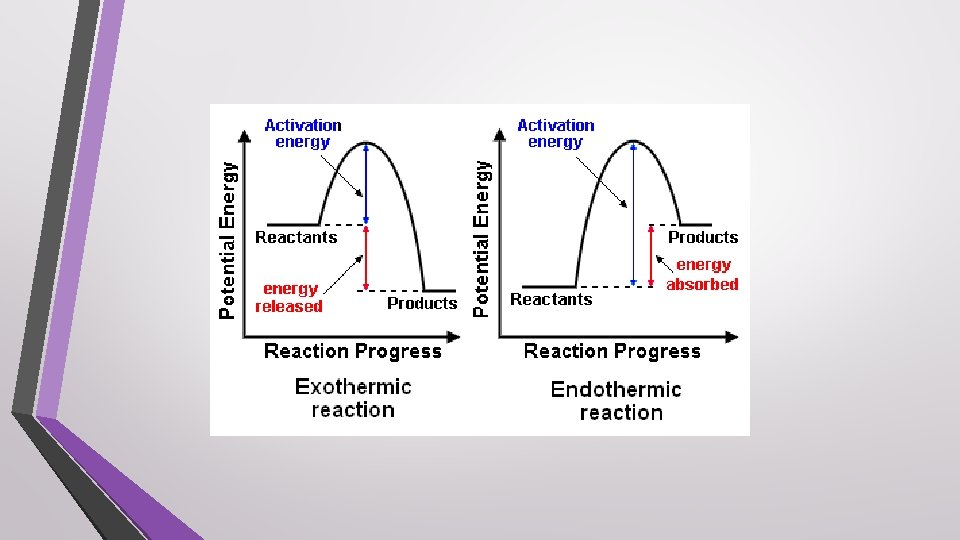
Endothermic Process • The system gains energy as the surroundings cool down

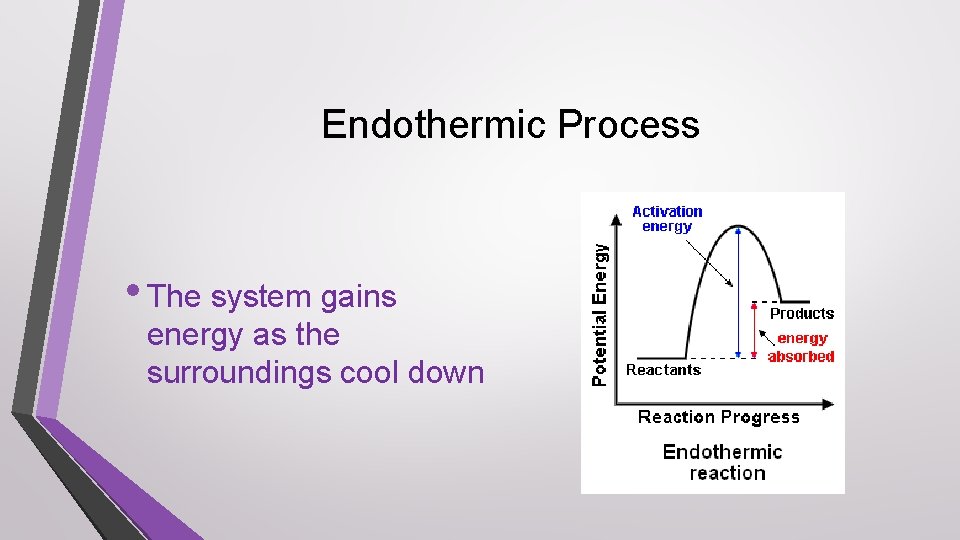
Exothermic Process • The system loses energy as the surroundings heat up


Units for Measuring Heat Flow • Calorie (cal) – the quantity of heat needed to raise the temperature of 1 g of pure water 1°C • Joule (J) – the quantity of heat needed to raise the temperature of 1 g of pure water 0. 2390°C • 1 J = 0. 2390 cal 4. 184 J = 1 cal

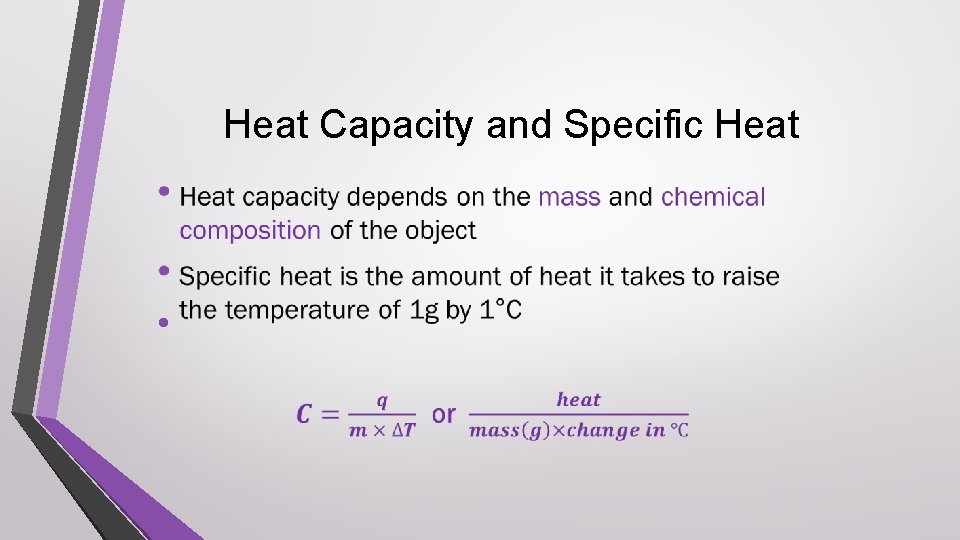
Heat Capacity and Specific Heat •

Calorimetry • The precise measurement of the heat flow into or out of a system for chemical or physical processes. • Enthalpy (H) of the system is the heat content of a system at constant pressure • A calorimeter is an insulated device used to measure the absorption or release of heat in chemical or physical processes • Constant-Pressure Calorimeters – foam cups which do not let much heat in or out, open to the atmosphere. • • Measures enthalpy (H), q = ∆H qsys = ∆H = -qsurr = -m x C x ∆T Negative ∆H values are exothermic reactions, while positive ∆H values are endothermic Constant-Volume Calorimeters, or bomb calorimeter

Enthalpy Calculation When 25 ml of water containing. 025 mol of HCl at 25°C is added to 25. 0 ml of water containing. 025 mol of Na. OH at 25°C in a calorimeter a reaction occurs. Calculate the enthalpy change in joules if the highest temperature observed is 32°C.

Thermochemical Equations • In a chemical equation, the enthalpy change for the reaction can be written as either a reactant or a product • A thermochemical equation includes the enthalpy change • The heat of reaction is the enthalpy change for the chemical equation exactly as it is written • The heat of combustion is the heat of reaction for the complete burning of one mole of a substance.

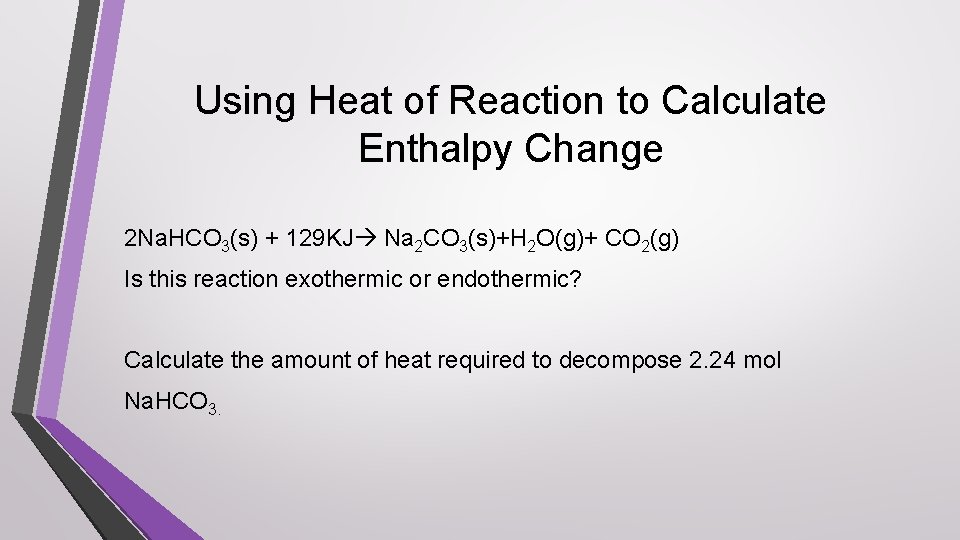
Using Heat of Reaction to Calculate Enthalpy Change 2 Na. HCO 3(s) + 129 KJ Na 2 CO 3(s)+H 2 O(g)+ CO 2(g) Is this reaction exothermic or endothermic? Calculate the amount of heat required to decompose 2. 24 mol Na. HCO 3.

Hess’s Law • Hess’s law allows you to determine the heat of reaction indirectly • Hess’s law of heat summation states that if you add two or more thermochemical equations to give a final equation, then you can also add the heats to give a final heat. • Useful in calculating the heat of formation for just one of multiple products

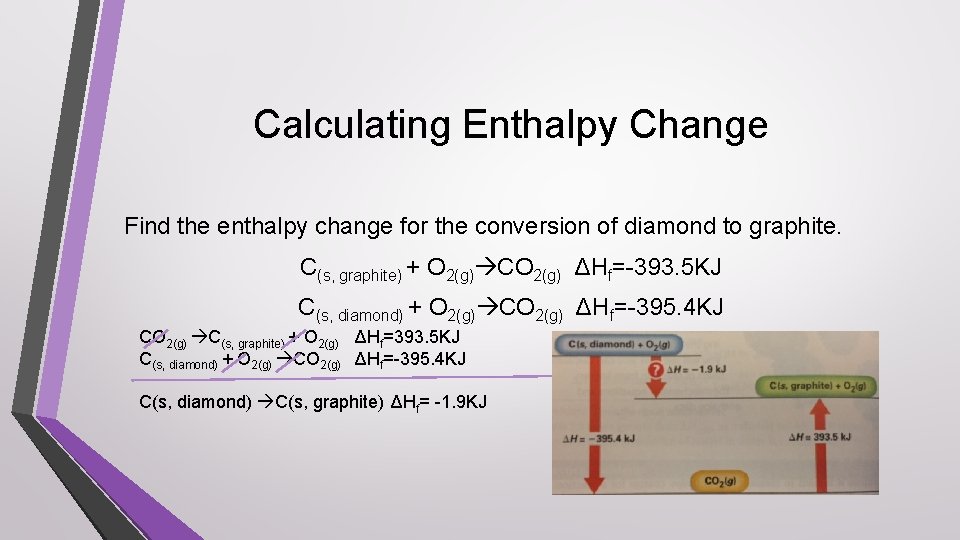
Calculating Enthalpy Change Find the enthalpy change for the conversion of diamond to graphite. C(s, graphite) + O 2(g) CO 2(g) ΔHf=-393. 5 KJ C(s, diamond) + O 2(g) CO 2(g) ΔHf=-395. 4 KJ CO 2(g) C(s, graphite) + O 2(g) ΔHf=393. 5 KJ C(s, diamond) + O 2(g) CO 2(g) ΔHf=-395. 4 KJ C(s, diamond) C(s, graphite) ΔHf= -1. 9 KJ

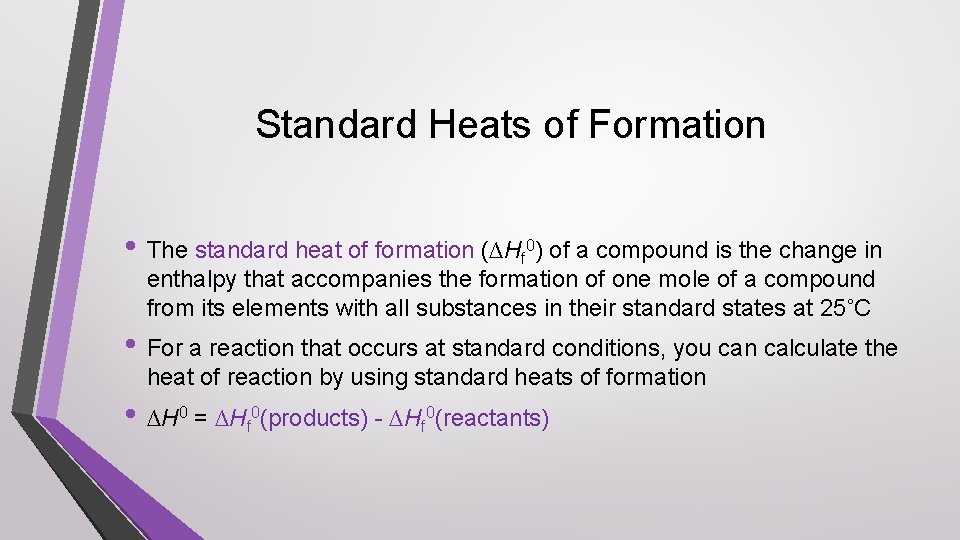
Standard Heats of Formation • The standard heat of formation (∆Hf 0) of a compound is the change in enthalpy that accompanies the formation of one mole of a compound from its elements with all substances in their standard states at 25˚C • For a reaction that occurs at standard conditions, you can calculate the heat of reaction by using standard heats of formation • ∆H 0 = ∆Hf 0(products) - ∆Hf 0(reactants)

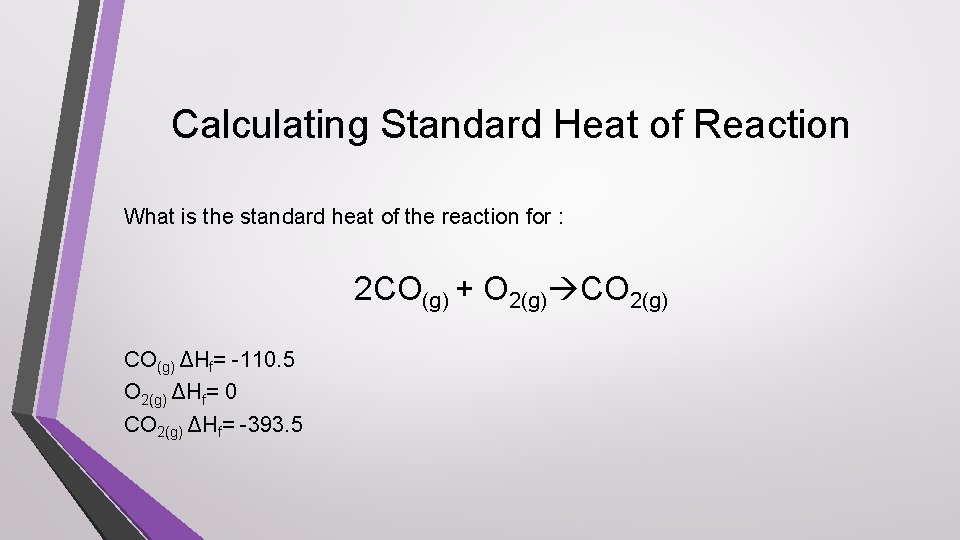
Calculating Standard Heat of Reaction What is the standard heat of the reaction for : 2 CO(g) + O 2(g) CO(g) ΔHf= -110. 5 O 2(g) ΔHf= 0 CO 2(g) ΔHf= -393. 5