Thermochemistry Calculations Heat Represented by q One effect
Thermochemistry Calculations

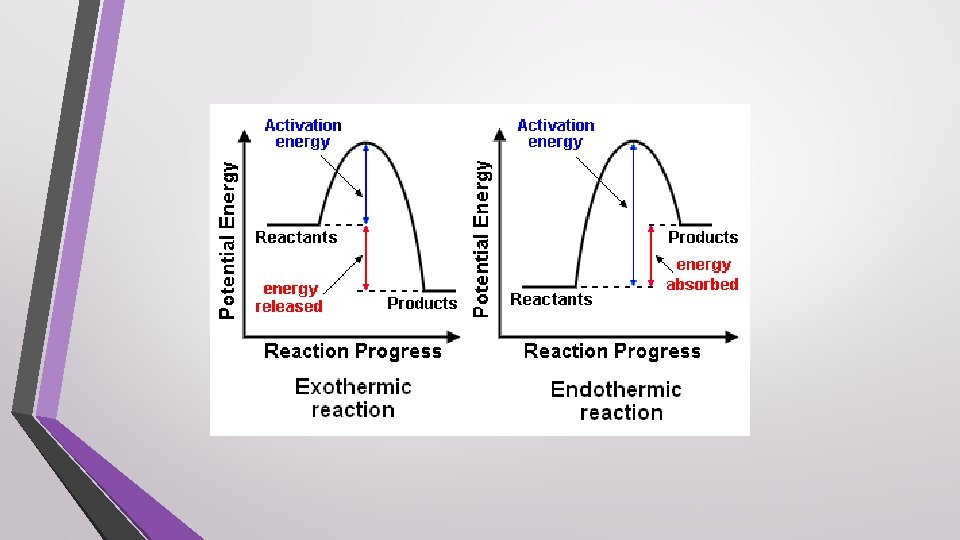
Heat • Represented by q • One effect of adding heat to an object is an increase in its temperature • Heat always flows from a warmer object to a cooler object

Processes • System – the part of the universe on which you focus your attention • Surroundings – everything else in the universe • Law of Conservation of Energy – in any chemical and physical process, energy is neither created or destroyed
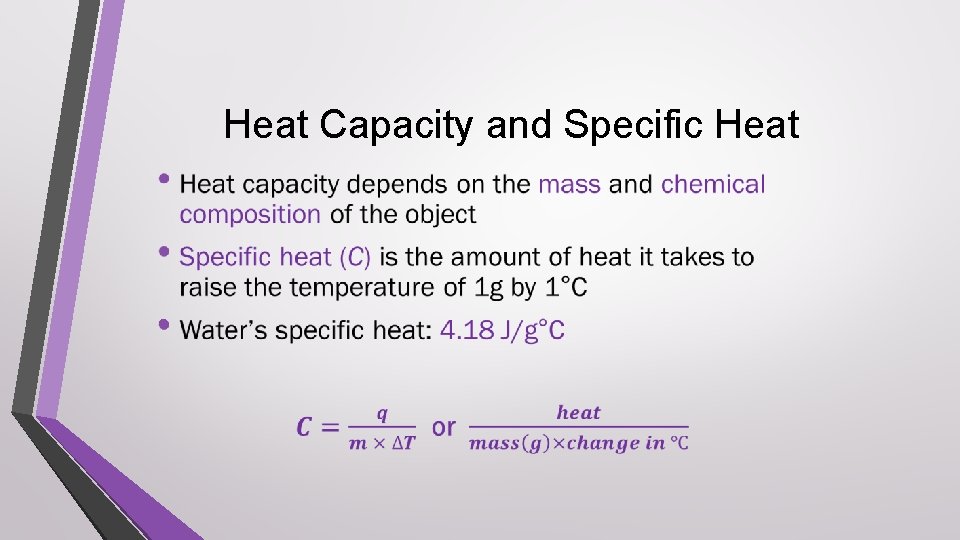
Heat Capacity and Specific Heat •

Calorimetry • Enthalpy (H) of the system is the heat content of a system at constant pressure • Measures enthalpy (H), q = ∆H • qsys = ∆H = -qsurr = m x C x ∆T • Negative ∆H values are exothermic reactions, while positive ∆H values are endothermic.

Enthalpy Calculation When 25 ml of water containing. 025 mol of HCl at 25°C is added to 25. 0 ml of water containing. 025 mol of Na. OH at 25°C in a calorimeter a reaction occurs. Calculate the enthalpy change in joules if the highest temperature observed is 32°C.
Thermochemical Equations • In a chemical equation, the enthalpy change for the reaction can be written as either a reactant or a product • A thermochemical equation includes the enthalpy change • The heat of reaction is the enthalpy change for the chemical equation exactly as it is written • The heat of combustion is the heat of reaction for the complete burning of one mole of a substance.
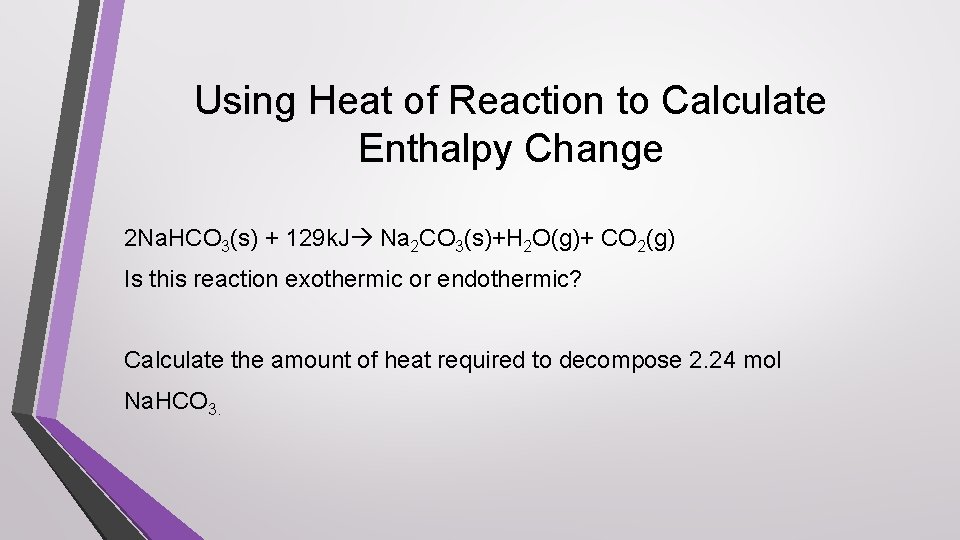
Using Heat of Reaction to Calculate Enthalpy Change 2 Na. HCO 3(s) + 129 k. J Na 2 CO 3(s)+H 2 O(g)+ CO 2(g) Is this reaction exothermic or endothermic? Calculate the amount of heat required to decompose 2. 24 mol Na. HCO 3.
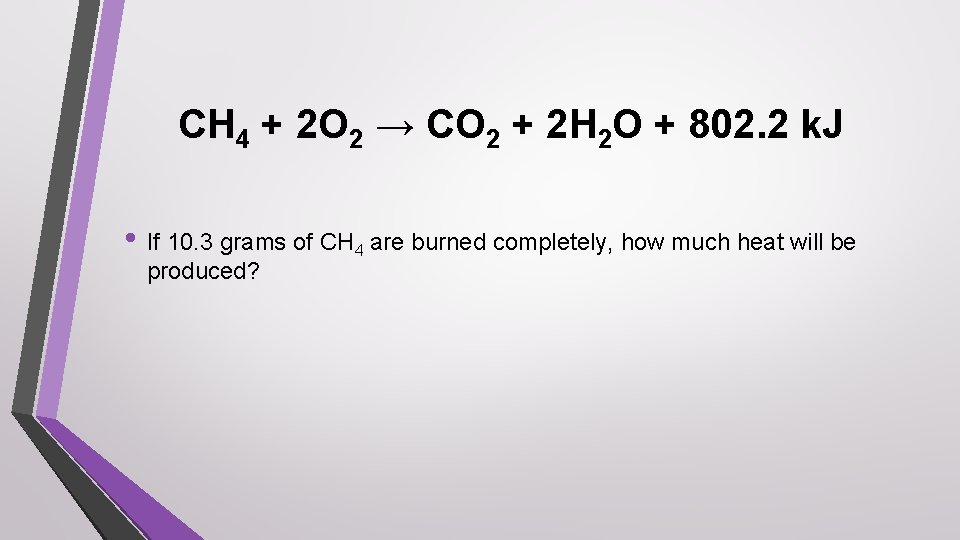
CH 4 + 2 O 2 → CO 2 + 2 H 2 O + 802. 2 k. J • If 10. 3 grams of CH 4 are burned completely, how much heat will be produced?

CH 4 + 2 O 2 → CO 2 + 2 H 2 O + 802. 2 k. J • How many liters of O 2 at STP would be required to produce 23 k. J of heat? • How many grams of water would be produced with 506 k. J of heat?

Heats of Fusion and Solidification • Molar Heat of Fusion ( Hfus) - the heat absorbed by one mole of a substance in melting from a solid to a liquid • Molar Heat of Solidification ( Hsolid) - heat lost when one mole of liquid solidifies

Heats of Vaporization and Condensation • Molar Heat of Vaporization ( Hvap) - the amount of heat necessary to vaporize one mole of a given liquid. • Molar Heat of Condensation ( Hcond) - amount of heat released when one mole of vapor condenses • Hvap = - Hcond
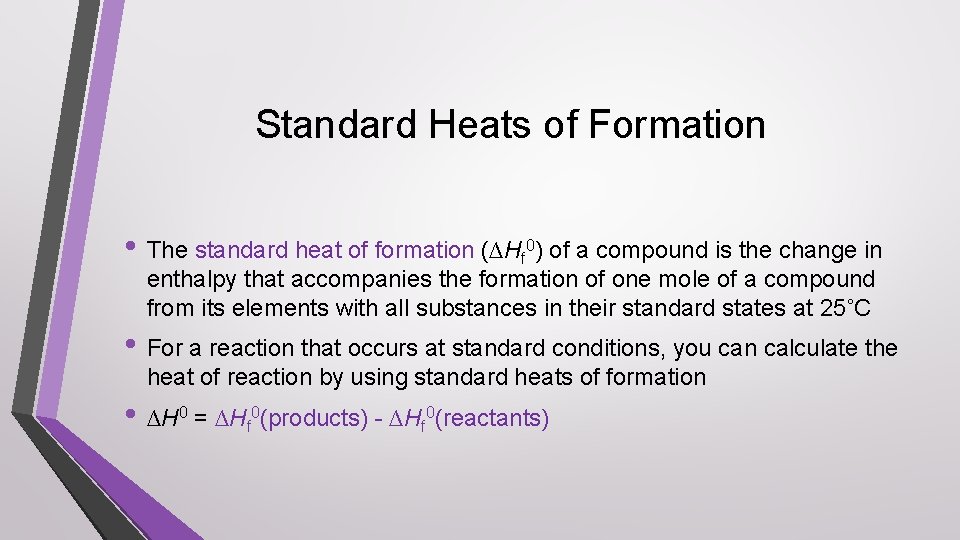
Standard Heats of Formation • The standard heat of formation (∆Hf 0) of a compound is the change in enthalpy that accompanies the formation of one mole of a compound from its elements with all substances in their standard states at 25˚C • For a reaction that occurs at standard conditions, you can calculate the heat of reaction by using standard heats of formation • ∆H 0 = ∆Hf 0(products) - ∆Hf 0(reactants)
- Slides: 14