Thermal Properties Mini Lesson In this lesson you


- Slides: 10

Thermal Properties Mini Lesson In this lesson, you will learn how different materials respond to heat. Remember, heat is the flow or transfer of thermal energy from one object to another. It happens by radiation, conduction or convection. Pay attention to 3 main ideas as you take notes: (1) conductors versus insulators (2) a property of matter called “specific heat” and (3) Thermal expansion

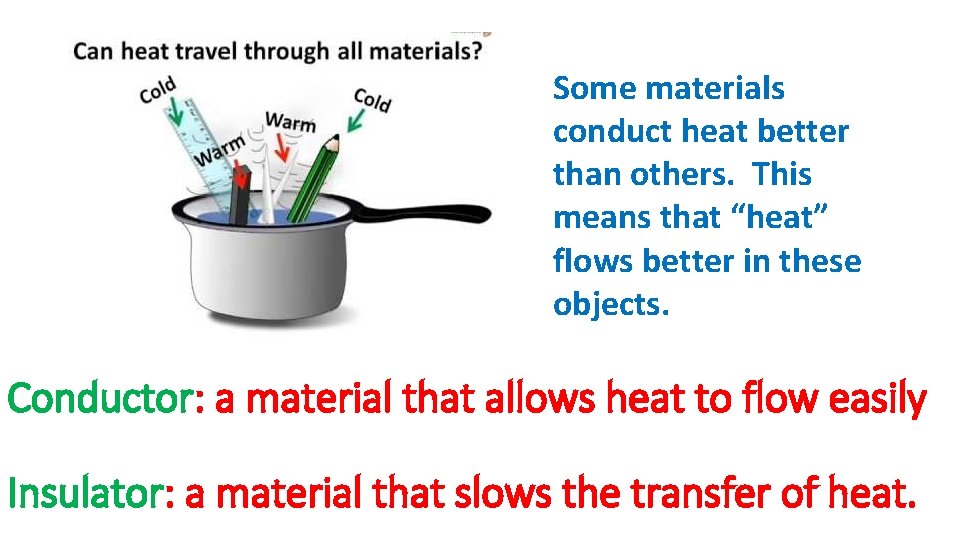
Some materials conduct heat better than others. This means that “heat” flows better in these objects. Conductor: a material that allows heat to flow easily Insulator: a material that slows the transfer of heat.

Metals make the best conductors. 1. List 3 insulators and 3 conductors from the Venn Diagram below.

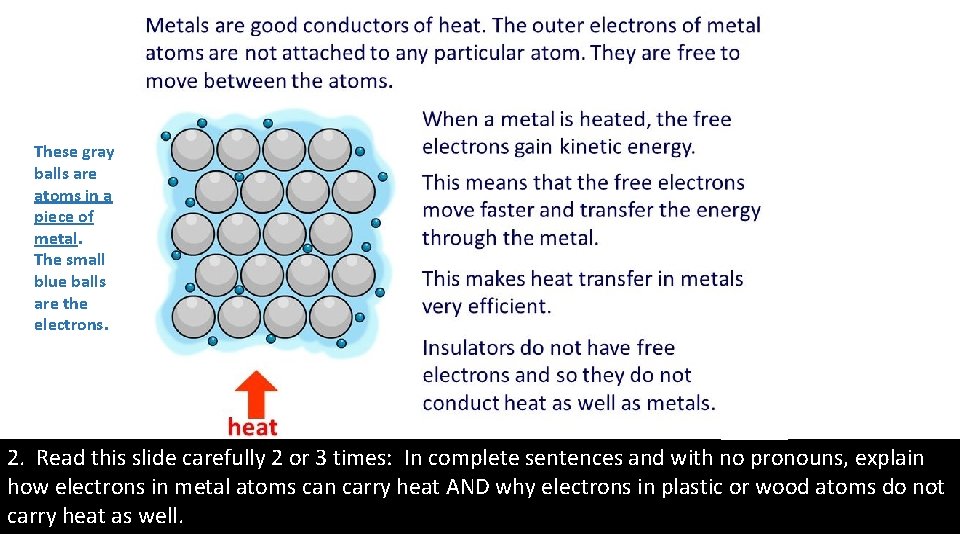
These gray balls are atoms in a piece of metal. The small blue balls are the electrons. 2. Read this slide carefully 2 or 3 times: In complete sentences and with no pronouns, explain how electrons in metal atoms can carry heat AND why electrons in plastic or wood atoms do not carry heat as well.

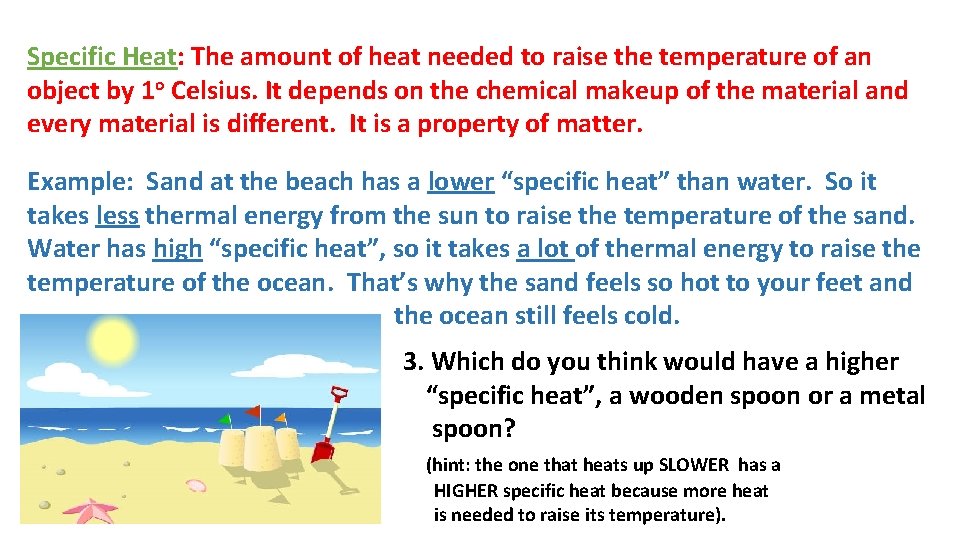
Specific Heat: The amount of heat needed to raise the temperature of an object by 1 o Celsius. It depends on the chemical makeup of the material and every material is different. It is a property of matter. Example: Sand at the beach has a lower “specific heat” than water. So it takes less thermal energy from the sun to raise the temperature of the sand. Water has high “specific heat”, so it takes a lot of thermal energy to raise the temperature of the ocean. That’s why the sand feels so hot to your feet and the ocean still feels cold. 3. Which do you think would have a higher “specific heat”, a wooden spoon or a metal spoon? (hint: the one that heats up SLOWER has a HIGHER specific heat because more heat is needed to raise its temperature).

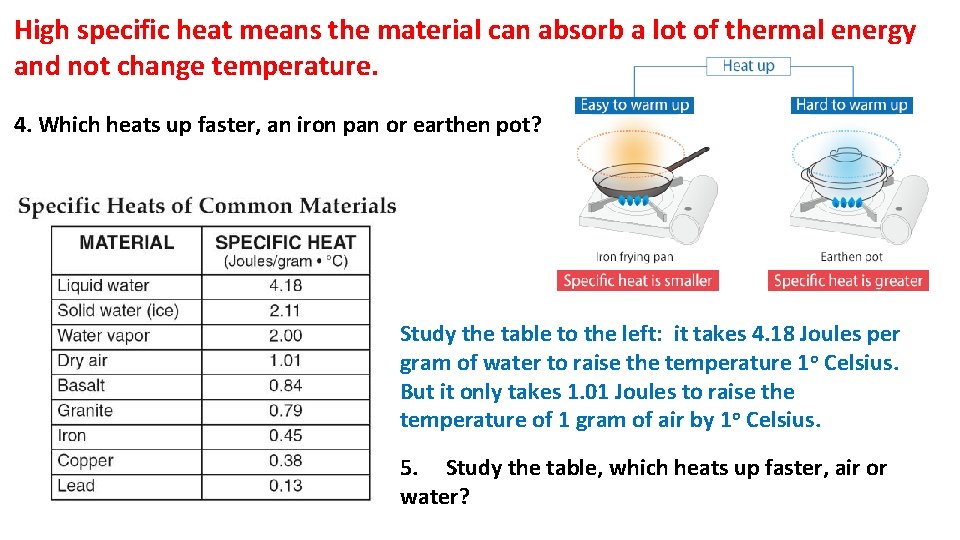
High specific heat means the material can absorb a lot of thermal energy and not change temperature. 4. Which heats up faster, an iron pan or earthen pot? Study the table to the left: it takes 4. 18 Joules per gram of water to raise the temperature 1 o Celsius. But it only takes 1. 01 Joules to raise the temperature of 1 gram of air by 1 o Celsius. 5. Study the table, which heats up faster, air or water?

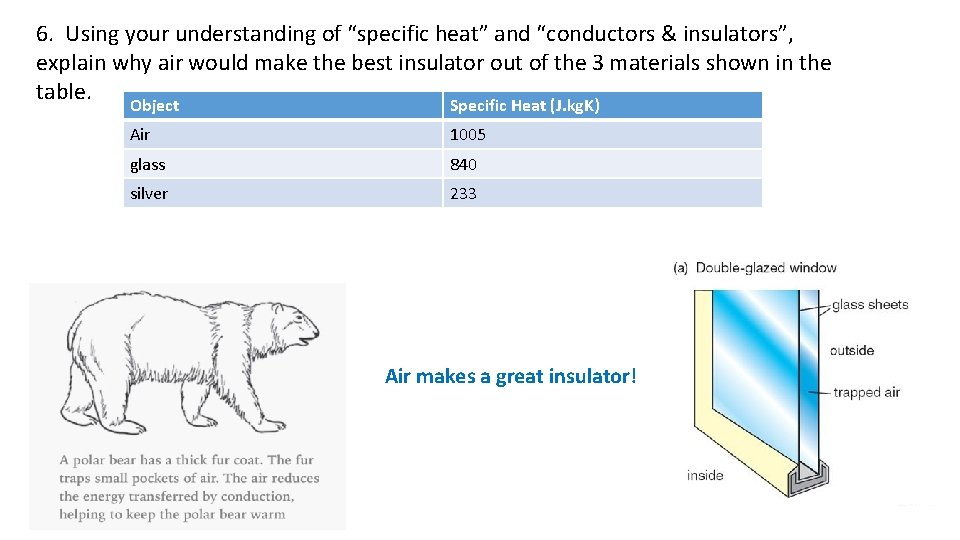
6. Using your understanding of “specific heat” and “conductors & insulators”, explain why air would make the best insulator out of the 3 materials shown in the table. Object Specific Heat (J. kg. K) Air 1005 glass 840 silver 233 Air makes a great insulator!

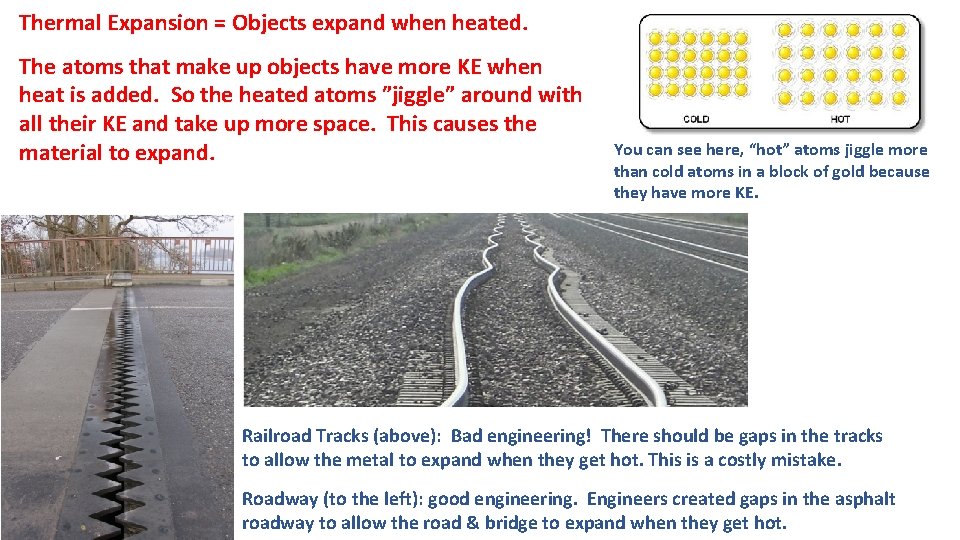
Thermal Expansion = Objects expand when heated. The atoms that make up objects have more KE when heat is added. So the heated atoms ”jiggle” around with all their KE and take up more space. This causes the material to expand. You can see here, “hot” atoms jiggle more than cold atoms in a block of gold because they have more KE. Railroad Tracks (above): Bad engineering! There should be gaps in the tracks to allow the metal to expand when they get hot. This is a costly mistake. Roadway (to the left): good engineering. Engineers created gaps in the asphalt roadway to allow the road & bridge to expand when they get hot.

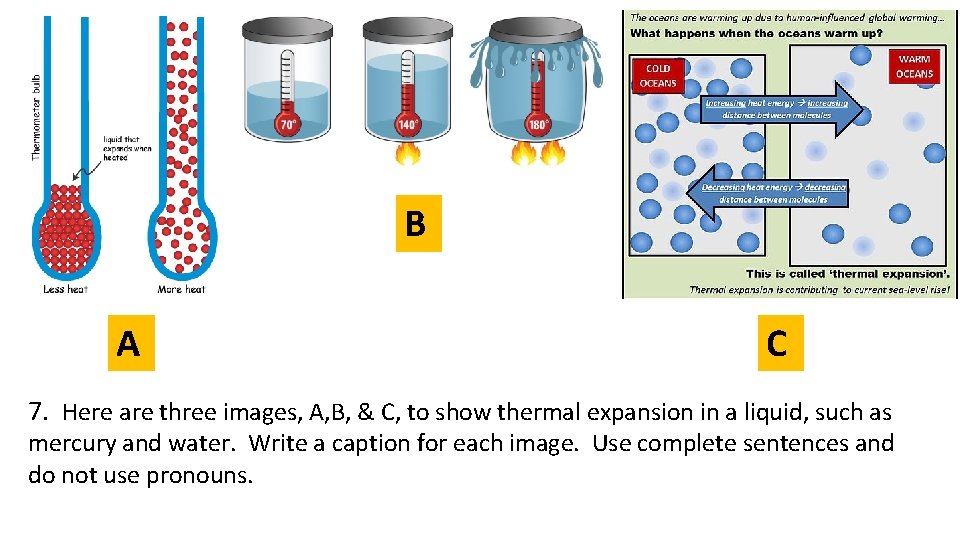
B A C 7. Here are three images, A, B, & C, to show thermal expansion in a liquid, such as mercury and water. Write a caption for each image. Use complete sentences and do not use pronouns.

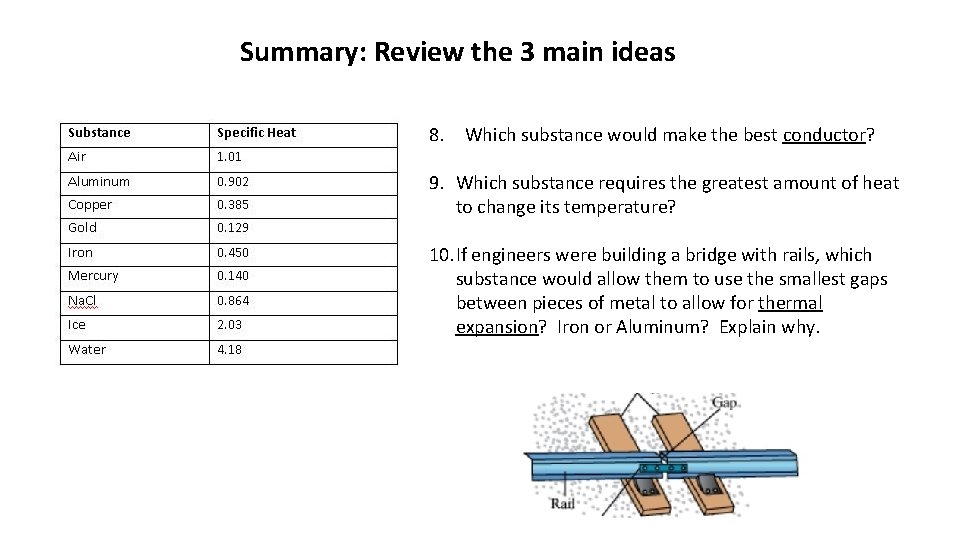
Summary: Review the 3 main ideas 8. Which substance would make the best conductor? 9. Which substance requires the greatest amount of heat to change its temperature? 10. If engineers were building a bridge with rails, which substance would allow them to use the smallest gaps between pieces of metal to allow for thermal expansion? Iron or Aluminum? Explain why.