THERMAL ENERGY THERMAL EQUILIBRIUM u Moves heat energy

THERMAL ENERGY

THERMAL EQUILIBRIUM u. Moves heat energy from warmer to cooler regions u. Example, water/antifreeze in a radiator
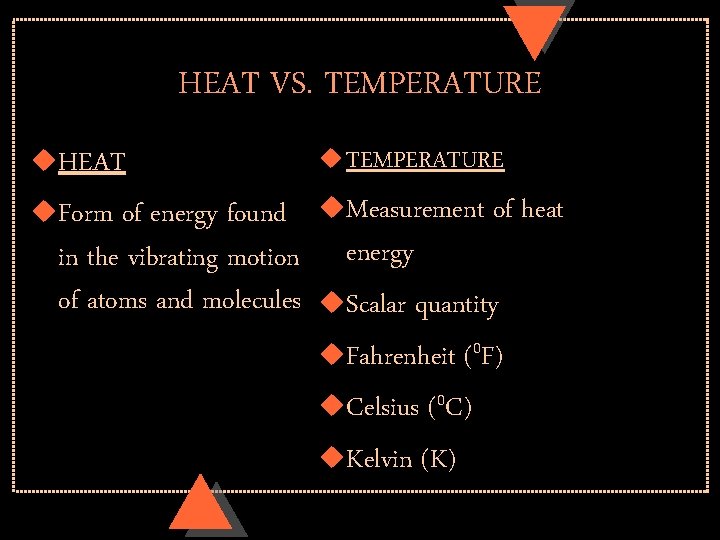
HEAT VS. TEMPERATURE u. HEAT u. Form of energy found in the vibrating motion of atoms and molecules u TEMPERATURE u. Measurement of heat energy u. Scalar quantity u. Fahrenheit (0 F) u. Celsius (0 C) u. Kelvin (K)

u. Heat increases the KE of the substance u. The substance expands especially gases u. Work may be done u. Examples- rubber band, steeel balls
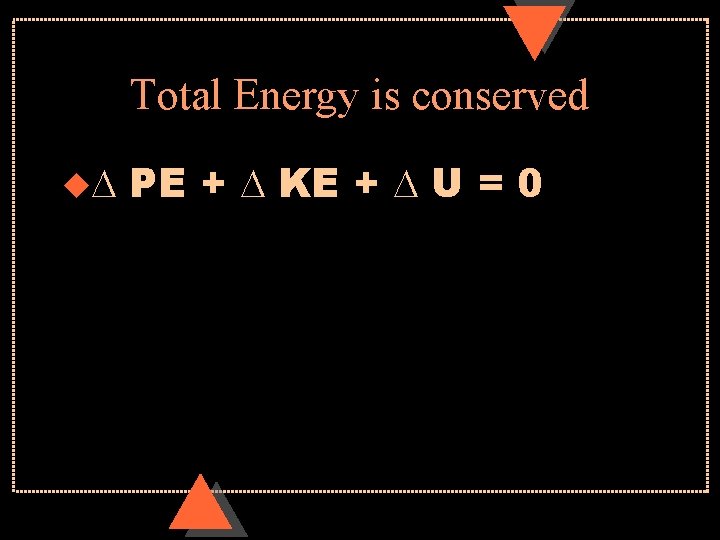
Total Energy is conserved u. D PE + D KE + D U = 0

WHY DOES HEAT FLOW FROM HOT- TO - COLD? u. Heat gives added energy to molecules which move faster and then the energy is passed on to surrounding cooler molecules
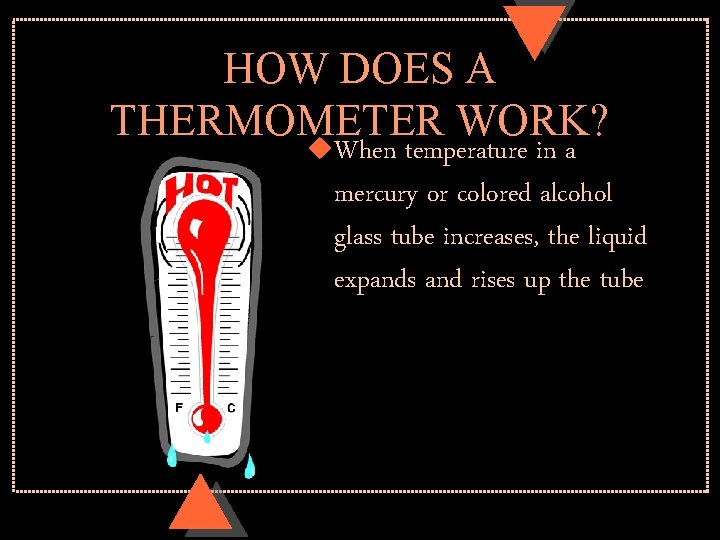
HOW DOES A THERMOMETER WORK? u. When temperature in a mercury or colored alcohol glass tube increases, the liquid expands and rises up the tube

TEMPERATURE SCALES u. Scale Boiling Pt. Freezing Pt. u. Fahrenheit 2120 F 320 F u. Celsius 1000 C 0 0 C u. Kelvin 373 K 273 K
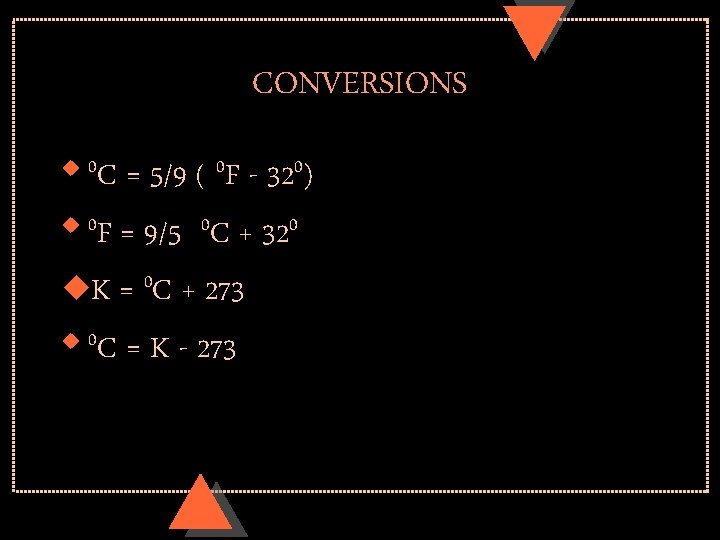
CONVERSIONS = 5/9 ( 0 F - 320) u 0 F = 9/5 0 C + 320 u. K = 0 C + 273 u 0 C = K - 273 u 0 C
SOLVE THIS PROBLEM An electric motor is operating at a temperature of 1150 F. It is located in a workshop where the room temperature is 750 F. u Find the room temperature in 0 C. u Find the room temperature in K. u

ROOM TEMPERATURE IN 0 C = 5/9 ( 75 -32) u 0 C = 5/9 ( 43 ) u 0 C = 23. 9 = 240 C u 0 C

ROOM TEMPERATURE TO K = 0 C + 273 u. K = 24 + 273 = 297 K u. K

Conduction, Convection, Radiation u Conduction- The transfer of energy through matter in which energy moves from particle to particle ---touching; more easily in solids than liquids or gases u Convection-The transfer of energy by the bulk movement of matter in which particles move from place to place in a fluid---often movement of heat in air u Radiation- The transfer of energy in the form of waves—often from the SUN
Conductors & Insulators u Thermal conductors-substances that rapidly transfer heat energy u Insulators reduces heat loss by slow transfer of heat energy

Specific Heat Capacity u. The quantity of heat required to raise the temperature of 1 kg of a substance by 10 C at constant pressure u. The specific heat of water is 4. 18 J / g. 0 C u

u. Q u = m c DT Q = heat measured in J c = specific heat capacity in J/kg. 0 C u u m = mass in kg u DT = temperature in 0 C u. Example- food lab
Phase Change u Melting or solidification u. Latent heat – energy per unit mass that is transferred during a phase change of a substance u. Can be heat of fusion or heat of vaporization

u Heat of fusion- energy transferred to change from solid to liquid or liquid to solid u Heat of vaporization- energy transferred to change from liquid to vapor or vapor to liquid u Vaporizing or condensing
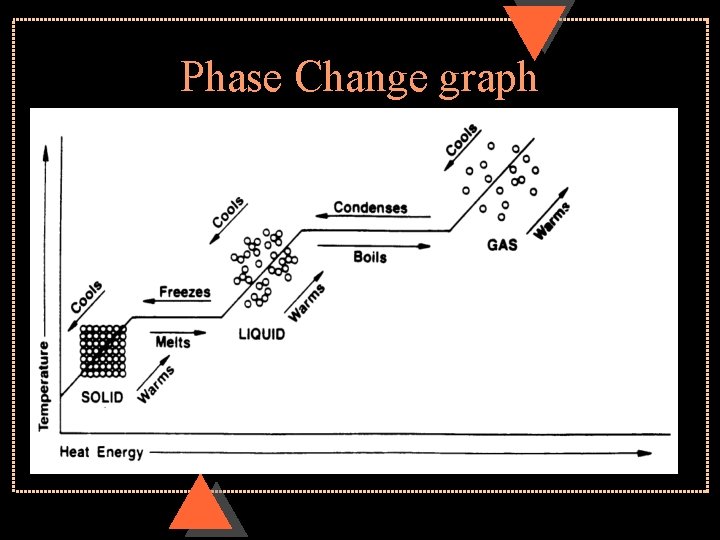
Phase Change graph
Laws of Thermodynamics u 1 st Law – The total change in internal energy from its initial to final equilibrium conditions is equal to the transfer of energy as both heat and work.

u 2 nd Law- No machine can transfer all of its absorbed energy as work u. This can be explained as entropy- a measure of the disorder of a system, natural processes increase entropy u. Increasing disorder means there is less energy to do work
- Slides: 21