Thermal Energy Heat Temperature Definitions Energy Can do








- Slides: 12

Thermal Energy Heat & Temperature

Definitions Energy Can do work Kinetic Energy associated with the motion of objects, large or small Thermal Kinetic Energy energy of microscopic particles that make up all matter

Definitions Heat Total amount of thermal energy an object possesses Flows from warmer to cooler objects (“Thermal energy in transit”) Temperature Average thermal energy of the molecules in a substance

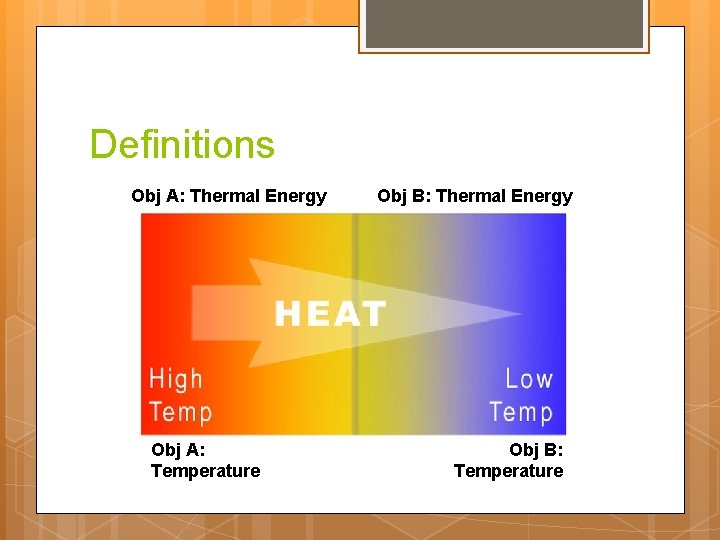
Definitions Obj A: Thermal Energy Obj A: Temperature Obj B: Thermal Energy Obj B: Temperature

Heat: Energy Units Calorie – amount of heat needed to change the temperature of 1 gram of water 1 C. Joule – (1 calorie = 4. 18 joules) NOTE: Food calories

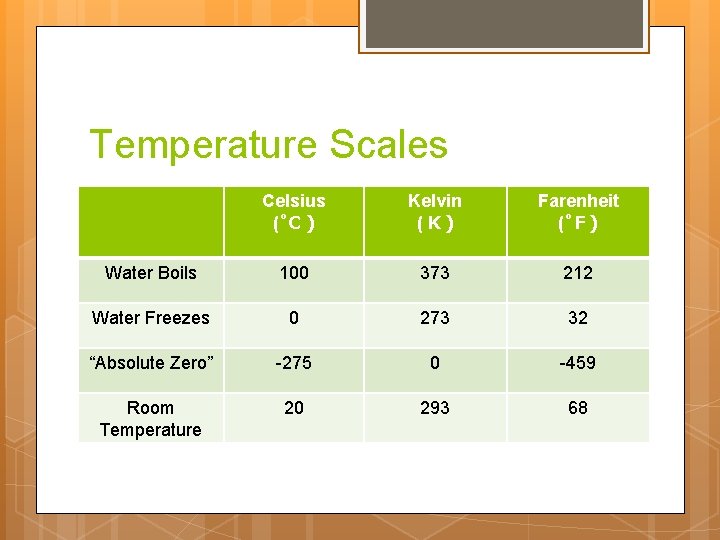
Temperature Scales Celsius ( C ): Our unit of choice Kelvin ( K ): Based on “absolute zero” Farenheit ( F ): Silly imperial unit

Temperature Scales Celsius ( C) Kelvin (K) Farenheit ( F) Water Boils 100 373 212 Water Freezes 0 273 32 “Absolute Zero” -275 0 -459 Room Temperature 20 293 68

Specific Heat Capacity Quantity of heat required to change the temperature of a unit mass of the substance by one degree. Measure of a substance’s ability to “store” thermal energy.

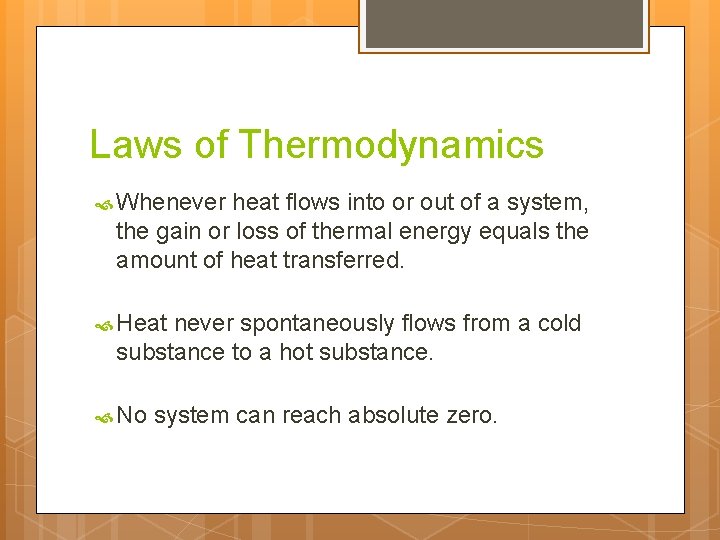
Laws of Thermodynamics Whenever heat flows into or out of a system, the gain or loss of thermal energy equals the amount of heat transferred. Heat never spontaneously flows from a cold substance to a hot substance. No system can reach absolute zero.

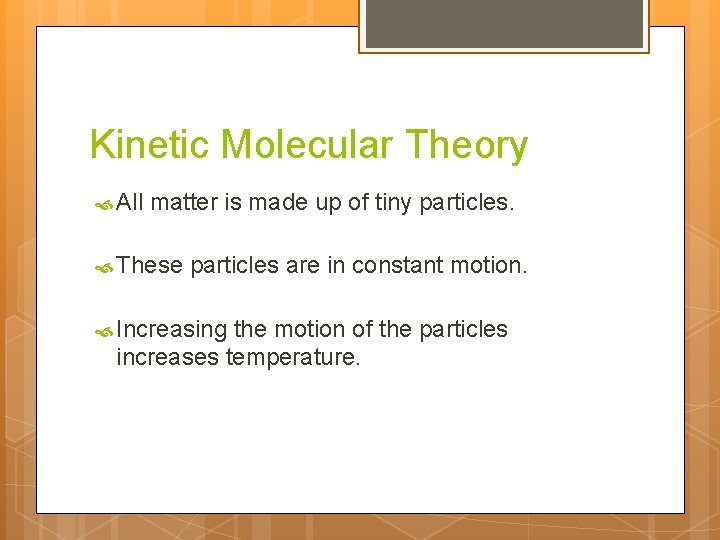
Kinetic Molecular Theory All matter is made up of tiny particles. These particles are in constant motion. Increasing the motion of the particles increases temperature.

Thermal Expansion As a substance is heated (thermal energy is increased), particles move faster and farther apart. Liquids expand more than solids with increases in temperature. Gases expand more than liquids with increases in temperature.

Definitions Kinetic energy is a general term describing the energy associated with the motion of objects (large or small objects). You can calculate the kinetic energy of an object of mass m with a velocity (speed) v from the formula K. E. = 1/2 mv^2. Thermal energy refers to the kinetic energy of the microscopic particles (atoms and molecules) that make up all samples of matter - i. e. all objects. When you add heat to an object, you increase the temperature of the object (usually) and that heat increases the kinetic energy of the molecules that comprise that object. In fact, temperature is a measure of the average kinetic energy of the microscopic particles that make up an object.