Thermal Energy Heat Temperature Definitions Energy Can do






- Slides: 20

Thermal Energy Heat & Temperature

Definitions Energy Can do work Kinetic Energy associated with the motion of objects, large or small Thermal Energy Kinetic energy of microscopic particles that make up all matter

Definitions Thermal Energy Total amount of thermal energy an object possesses Temperature Average thermal energy per molecule “Concentration” of thermal energy in an object With the same total amount of thermal energy, a larger object has a lower temperature. Heat Amount of thermal energy that is transmitted from body to body. “Thermal energy in transit”

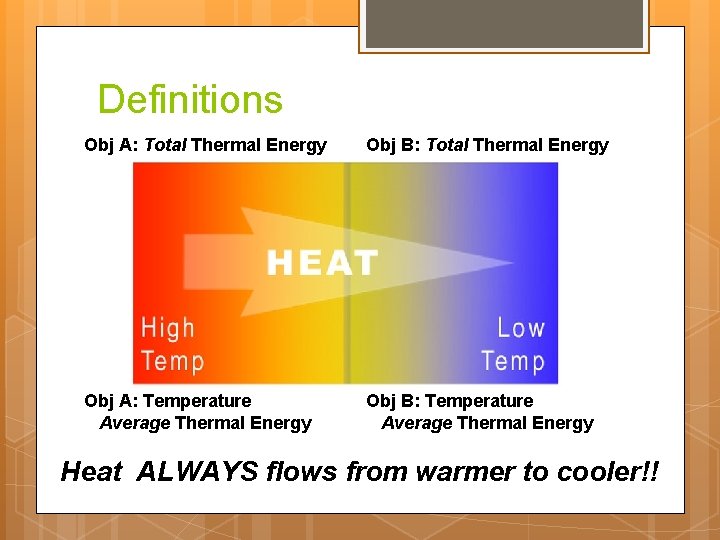
Definitions Obj A: Total Thermal Energy Obj B: Total Thermal Energy Obj A: Temperature Average Thermal Energy Obj B: Temperature Average Thermal Energy Heat ALWAYS flows from warmer to cooler!!

Definitions: HEAT - continues Amount of thermal energy that is transmitted from body to body. “Thermal energy in transit” Thermal energy is money in the bank Heat is the transfer of the money in and out of the bank Flows from hotter to colder until both have the same temperature (THERMAL EQUILIBRIUM) Body does not “have” a certain amount of heat. It “has” a certain amount of thermal energy, and then transfers heat to another body.

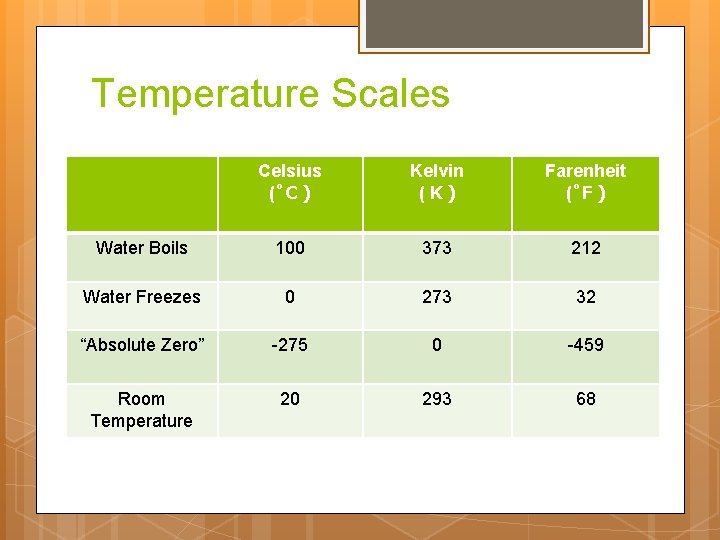
Units: Temperature Scales Celsius ( C ): Our unit of choice Kelvin ( K ): Based on “absolute zero” Molecules have NO motion (Zero kinetic / thermal energy) Farenheit ( F ): Silly imperial unit

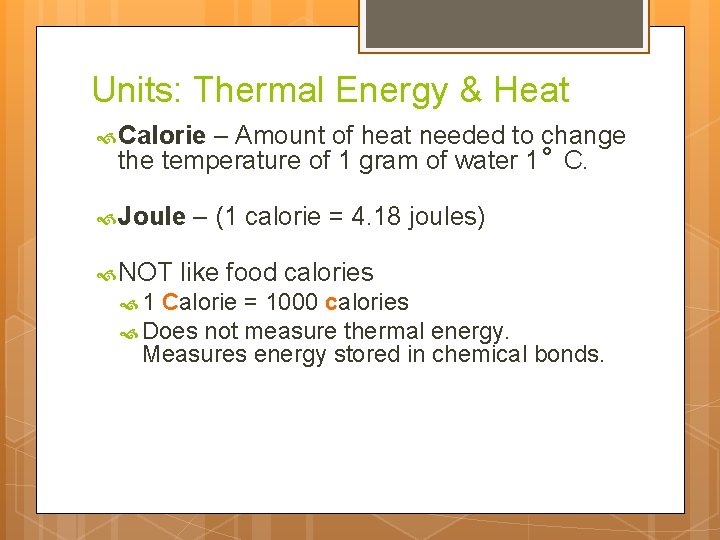
Units: Thermal Energy & Heat Calorie – Amount of heat needed to change the temperature of 1 gram of water 1 C. Joule – (1 calorie = 4. 18 joules) NOT like food calories 1 Calorie = 1000 calories Does not measure thermal energy. Measures energy stored in chemical bonds.

Temperature Scales Celsius ( C) Kelvin (K) Farenheit ( F) Water Boils 100 373 212 Water Freezes 0 273 32 “Absolute Zero” -275 0 -459 Room Temperature 20 293 68

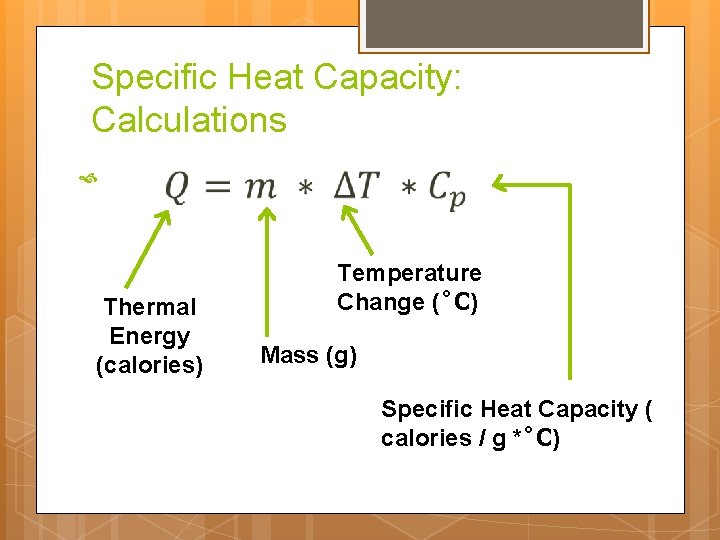
Specific Heat Capacity Quantity of heat required to change the temperature of a unit mass of the substance by one degree. ex: water = 1 calorie / (gram)( C) Relationship between heat and temperature How much heat does it take to increase the temperature

Specific Heat Capacity “Specific” – Means “per unit mass” Capacity - Measure of a substance’s ability to “store” thermal energy. Like a person’s “capacity” for alcohol How much alcohol he/she can “store” before there is a change in behavior.

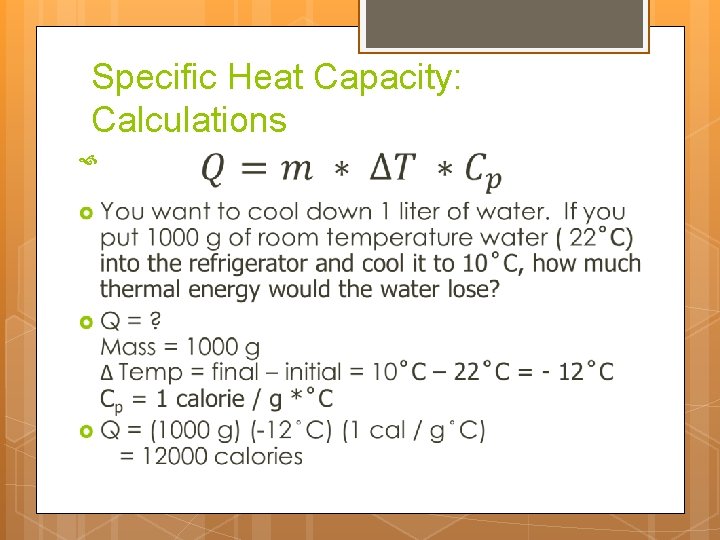
Specific Heat Capacity: Calculations Thermal Energy (calories) Temperature Change ( C) Mass (g) Specific Heat Capacity ( calories / g * C)

Specific Heat Capacity: Calculations

Laws of Thermodynamics: “The movement of heat” When heat flows into or out of a system, the gain or loss of thermal energy equals the amount of heat transferred. (Conservation of energy) Heat never spontaneously flows from a cold substance to a hot substance. No system can reach absolute zero.

Kinetic Molecular Theory All matter is made up of tiny particles. These particles are in constant motion. Increasing the motion of the particles increases temperature. When particles collide, they transfer energy – the higher energy particle transfers energy to the lower energy particle. BUT… there is no overall loss of energy.

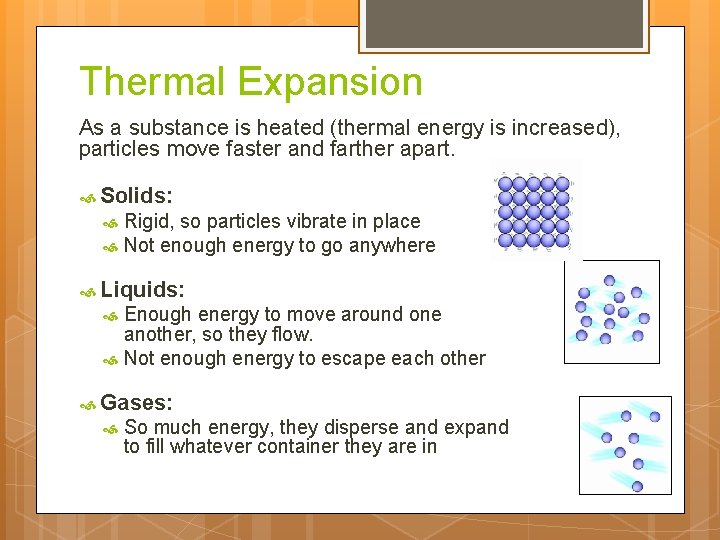
Thermal Expansion As a substance is heated (thermal energy is increased), particles move faster and farther apart. Solids: Rigid, so particles vibrate in place Not enough energy to go anywhere Liquids: Enough energy to move around one another, so they flow. Not enough energy to escape each other Gases: So much energy, they disperse and expand to fill whatever container they are in

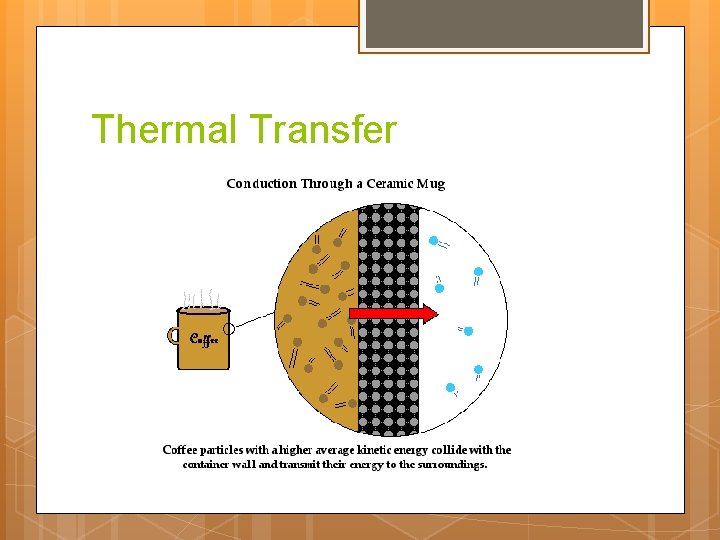
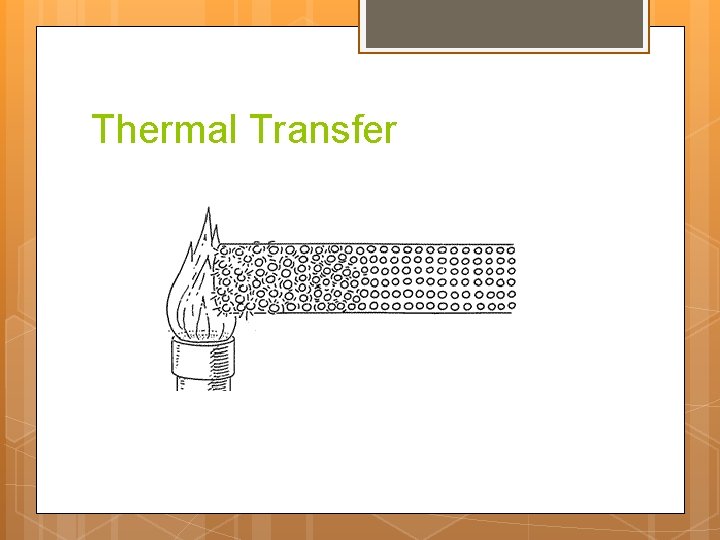
Thermal Transfer

Thermal Transfer


Kinetic Molecular Theory All matter is made up of tiny particles. These particles are in constant motion. Increasing the motion of the particles increases temperature. Thermodynamics Whenever heat flows into or out of a system, the gain or loss of thermal energy equals the amount of heat transferred. Heat never spontaneously flows from a cold substance to a hot substance. No system can reach absolute zero.

Definitions Kinetic energy is a general term describing the energy associated with the motion of objects (large or small objects). You can calculate the kinetic energy of an object of mass m with a velocity (speed) v from the formula K. E. = 1/2 mv^2. Thermal energy refers to the kinetic energy of the microscopic particles (atoms and molecules) that make up all samples of matter - i. e. all objects. When you add heat to an object, you increase the temperature of the object (usually) and that heat increases the kinetic energy of the molecules that comprise that object. In fact, temperature is a measure of the average kinetic energy of the microscopic particles that make up an object.