Thermal Energy and Heat Temperature Particle level kinetic
















- Slides: 33

Thermal Energy and Heat

Temperature • Particle level kinetic energy • A measure of the average kinetic energy of the atoms or molecules of a substance • Temperature increases if the motion of the particles increases

Thermal Energy (Q) • Particle level Mechanical (total) Energy • Total kinetic and potential energy of a system’s particles

Heat • Particle level WORK • The transfer of thermal energy

Methods of Heat Transfer Conduction • Heat transfer through a material by collision of atoms • Particles in flame gain kinetic energy and start moving faster – they collide with neighbouring atoms making them move more rapidly and heat spreads • Occurs well in metals

Methods of Heat Transfer Convection • the process of transferring heat by a circulating path of fluid particles – called a convection current • Hot fluid spreads out and moves upwards, cooler fluid takes its place creating a current

Methods of Heat Transfer Radiation • Energy transfer by electromagnetic waves – no particles are necessary • i. e. visible, radio, micro, UV, infrared, X-rays

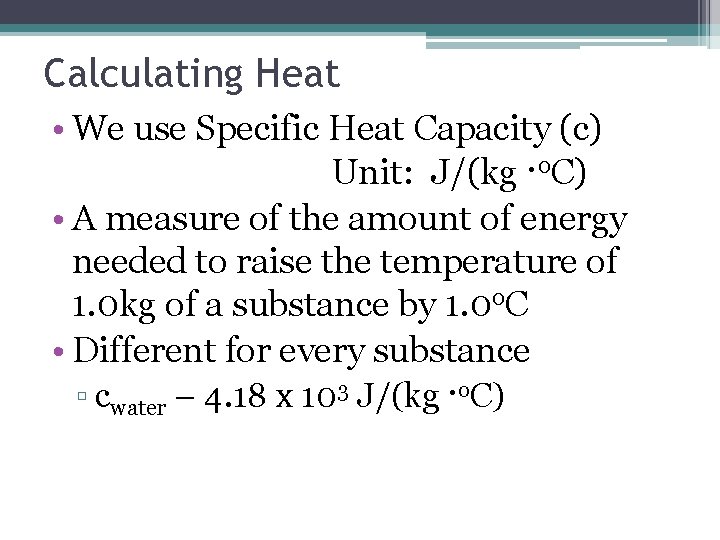
Calculating Heat • We use Specific Heat Capacity (c) Unit: J/(kg ·o. C) • A measure of the amount of energy needed to raise the temperature of 1. 0 kg of a substance by 1. 0 o. C • Different for every substance ▫ cwater – 4. 18 x 103 J/(kg ·o. C)

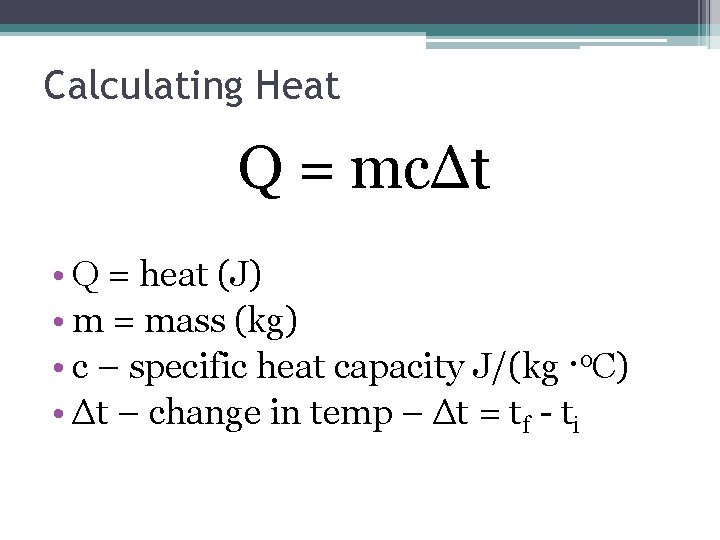
Calculating Heat Q = mcΔt • Q = heat (J) • m = mass (kg) • c – specific heat capacity J/(kg ·o. C) • Δt – change in temp – Δt = tf - ti

Ex 1: What is the mass of a bucket of water that requires 8. 4 x 104 J of heat to increase its temperature from 12 o. C to 22 o. C? Ans: m = 2. 0 kg

Latent Heat

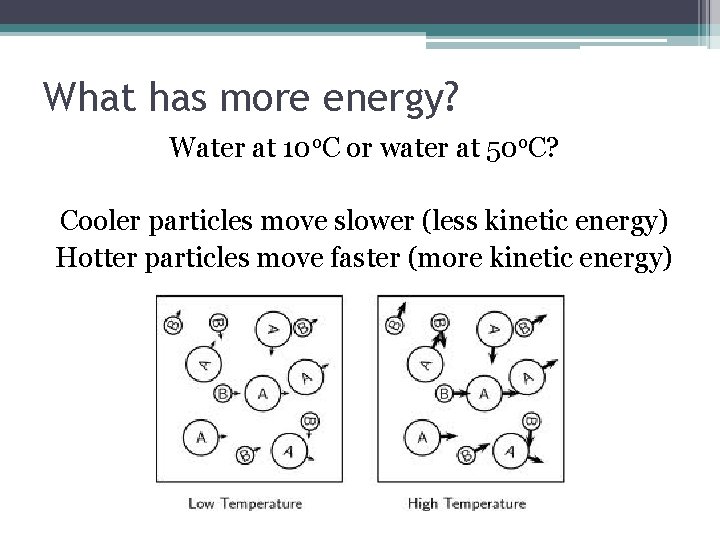
What has more energy? Water at 10 o. C or water at 50 o. C? Cooler particles move slower (less kinetic energy) Hotter particles move faster (more kinetic energy)

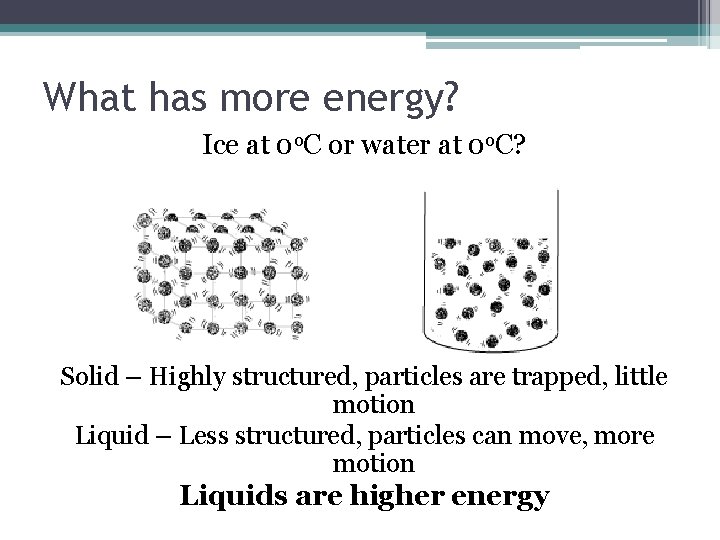
What has more energy? Ice at 0 o. C or water at 0 o. C? Solid – Highly structured, particles are trapped, little motion Liquid – Less structured, particles can move, more motion Liquids are higher energy

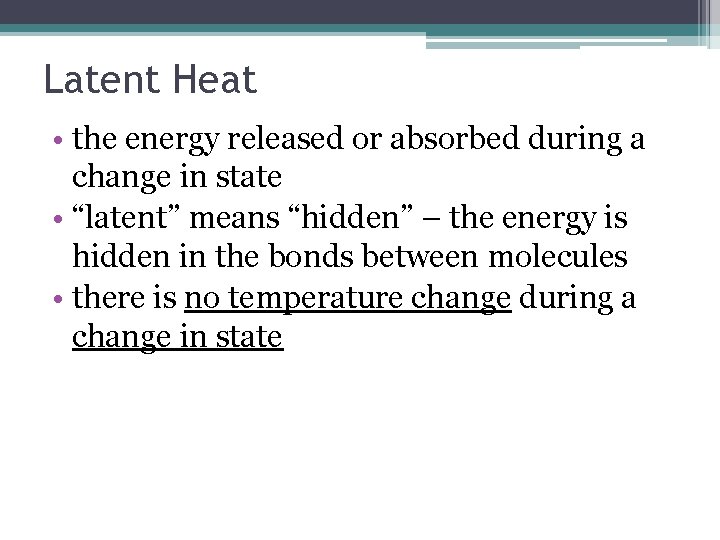
Latent Heat • the energy released or absorbed during a change in state • “latent” means “hidden” – the energy is hidden in the bonds between molecules • there is no temperature change during a change in state

Specific Latent Heat – the heat required for a particular mass of substance to change state

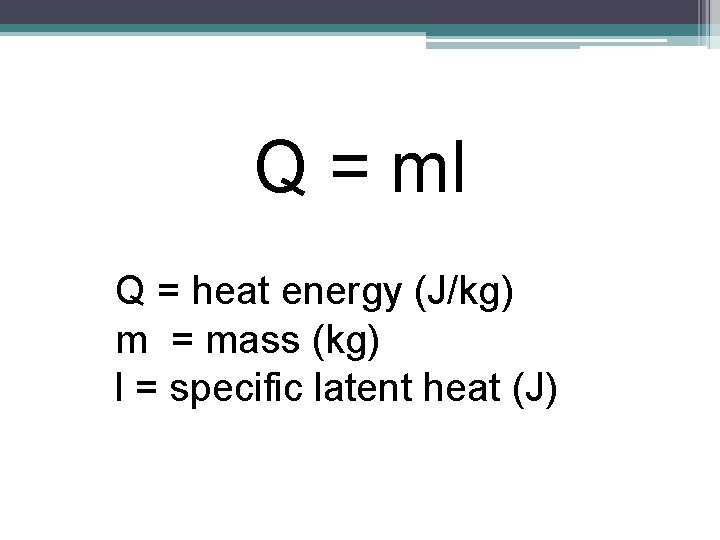
Q = ml Q = heat energy (J/kg) m = mass (kg) l = specific latent heat (J)

Specific Heat of Fusion • the quantity of heat required to melt/freeze 1 kg of a substance without changing the temperature

Specific Heat of Vaporization • the quantity of heat required to vaporize/condense 1 kg of a substance without changing the temperature

Using the latent heat values from your sheet: How much energy is required to turn 29. 0 g of solid oxygen at -219 o. C into liquid oxygen at 219 o. C? Ans: Q = 403 J How much energy is required to turn a 50 g, 0 o. C ice cube into 0 o. C water? Ans: Q = 1. 7 x 104 J

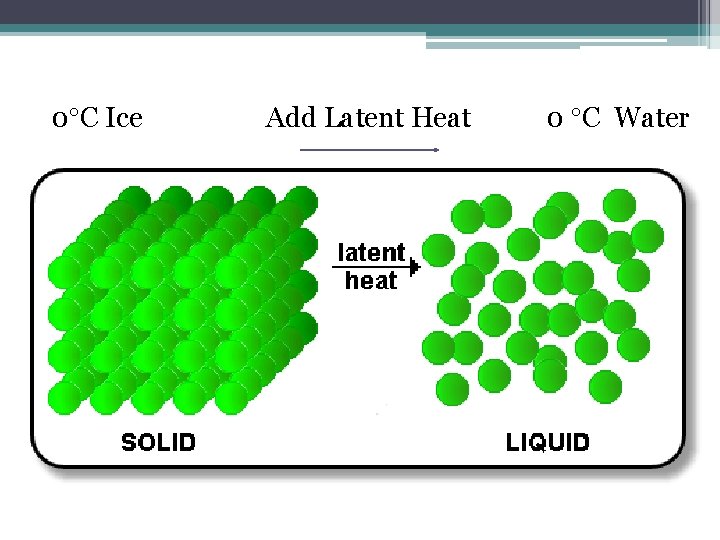
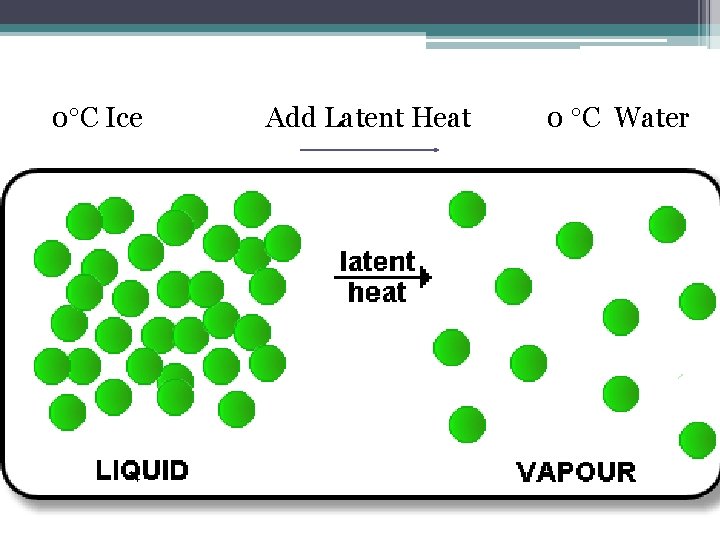
Consider an ice cube melting • Below 0 o. C the ice is solid, heat energy from the surroundings increases the temperature of the ice • As the ice melts, the heat energy is used to break the forces of attraction that hold the ice in a solid crystal lattice (no temperature change) • Once the ice is melted, the heat energy from the surroundings can be used to increase the temperature of the water

0 C Ice Add Latent Heat 0 C Water

Consider an ice cube melting • As the water reaches 100 o. C, the heat energy will stop raising the temperature and be used to break the forces of attraction in order to turn the liquid into a gas

0 C Ice Add Latent Heat 0 C Water

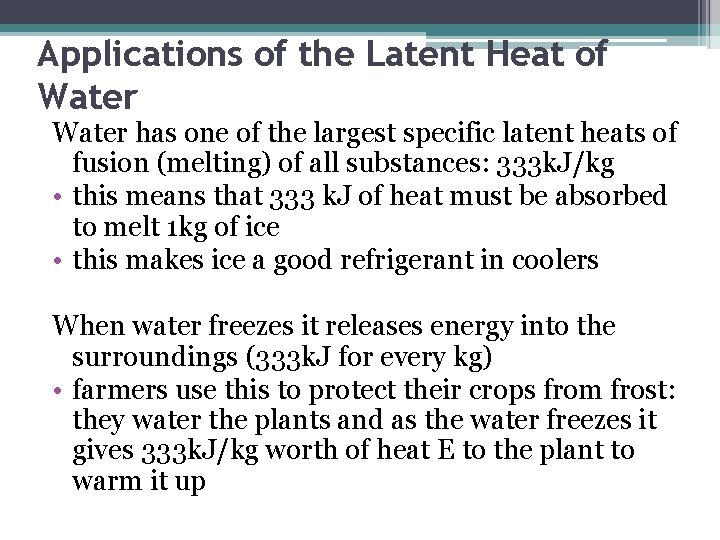
Applications of the Latent Heat of Water has one of the largest specific latent heats of fusion (melting) of all substances: 333 k. J/kg • this means that 333 k. J of heat must be absorbed to melt 1 kg of ice • this makes ice a good refrigerant in coolers When water freezes it releases energy into the surroundings (333 k. J for every kg) • farmers use this to protect their crops from frost: they water the plants and as the water freezes it gives 333 k. J/kg worth of heat E to the plant to warm it up

Applications of the Latent Heat of Water A burn from steam is worse than a burn from boiling water! • when water condense on a person's skin, 2268 J of energy is transferred to the skin

Sample Problem: • How much energy is required to turn 60 g of 15 o. C ice into 50 o. C water? -

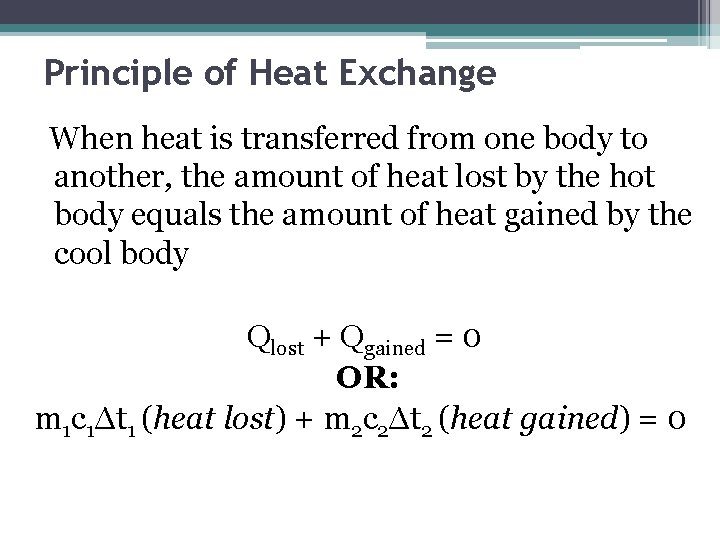
Principle of Heat Exchange When heat is transferred from one body to another, the amount of heat lost by the hot body equals the amount of heat gained by the cool body Qlost + Qgained = 0 OR: m 1 c 1Δt 1 (heat lost) + m 2 c 2Δt 2 (heat gained) = 0

Principle of Heat Exchange • When heat is transferred from one object to another, it usually flows from the hotter object to the cooler one

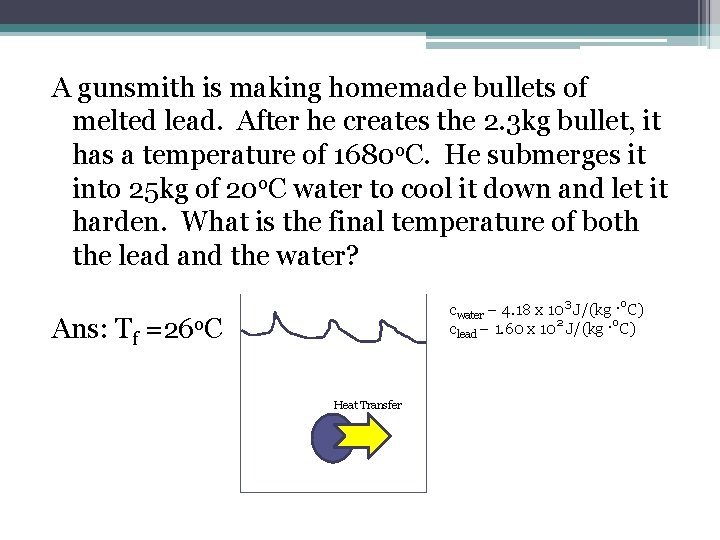
A gunsmith is making homemade bullets of melted lead. After he creates the 2. 3 kg bullet, it has a temperature of 1680 o. C. He submerges it into 25 kg of 20 o. C water to cool it down and let it harden. What is the final temperature of both the lead and the water? cwater – 4. 18 x 103 J/(kg ·o. C) clead – 1. 60 x 102 J/(kg ·o. C) Ans: Tf =26 o. C Heat Transfer

Solution

More on latent heat:

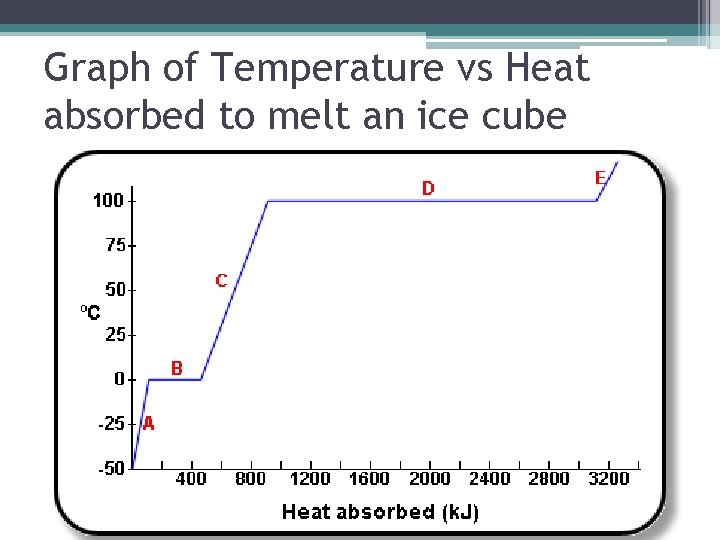
Graph of Temperature vs Heat absorbed to melt an ice cube

• A: Heat is absorbed by the surroundings to raise the temp. of the ice • B: Temperature doesn't change – energy is used to break forces of attraction within the solid to turn it into a liquid • C: Heat is absorbed by the surroundings to raise the temp of the water. • D: Temperature doesn't change – energy is used to break forces of attraction within the liquid to turn it into a gas • E: Heat is absorbed by the surroundings to raise the temperature of the gas