Thermal Energy and Heat Bell Ringer What do

Thermal Energy and Heat

Bell Ringer �What do you think thermal energy is?
Section 1: Temperature and Thermal Energy
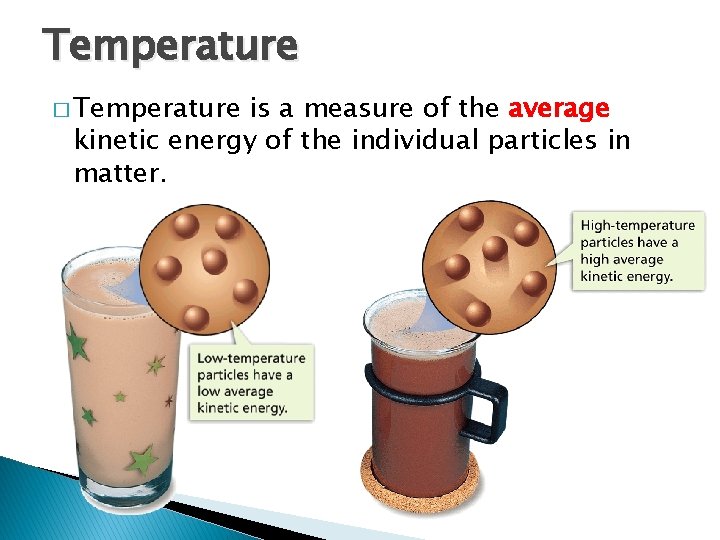
Temperature � Temperature, Thermal Energy and Heat is a measure of the average kinetic energy of the individual particles in matter.
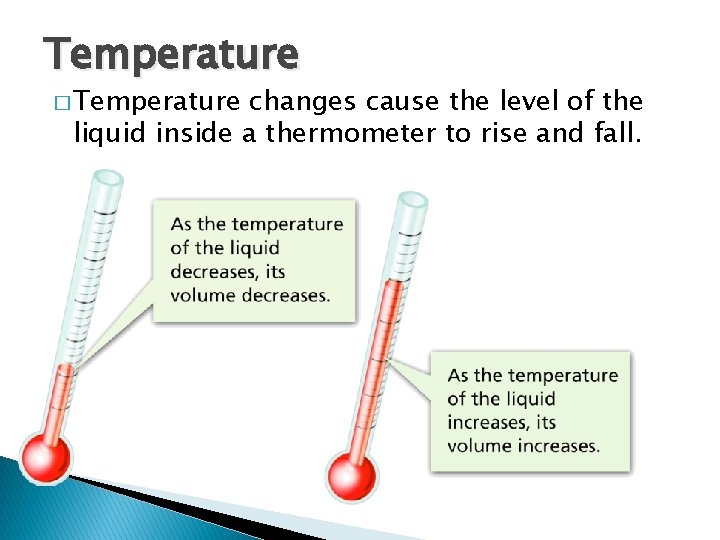
Temperature � Temperature, Thermal Energy and Heat changes cause the level of the liquid inside a thermometer to rise and fall.
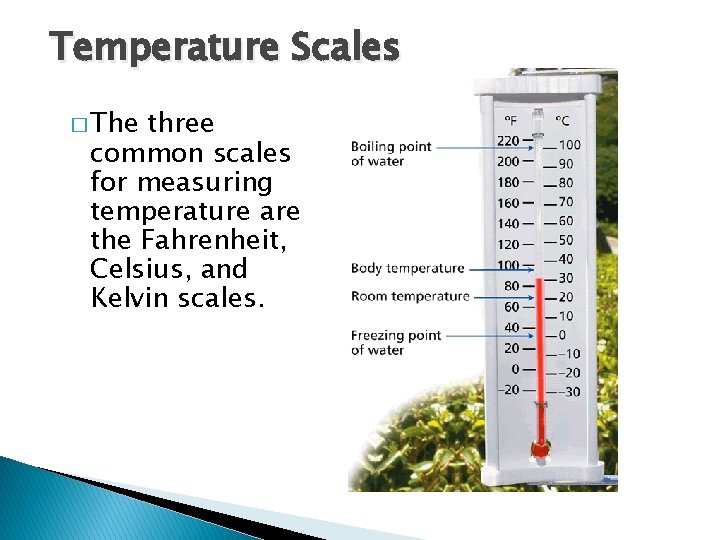
Temperature, Thermal Energy and Heat Temperature Scales � The three common scales for measuring temperature are the Fahrenheit, Celsius, and Kelvin scales.
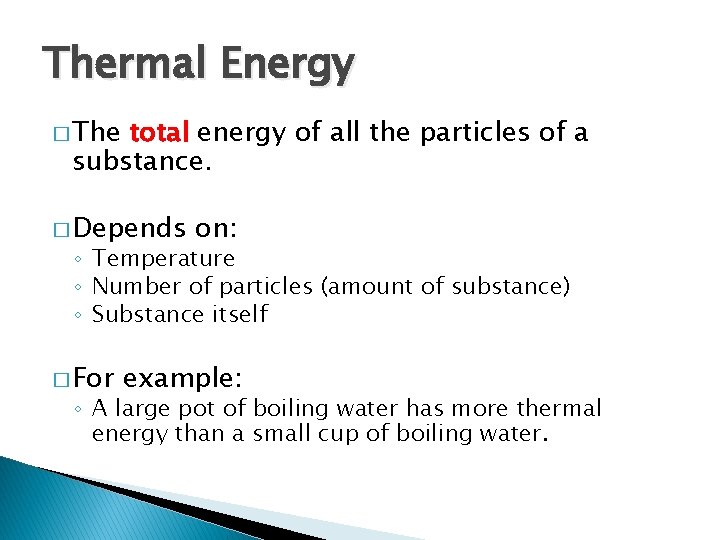
Thermal Energy � The total energy of all the particles of a substance. � Depends on: ◦ Temperature ◦ Number of particles (amount of substance) ◦ Substance itself � For example: ◦ A large pot of boiling water has more thermal energy than a small cup of boiling water.
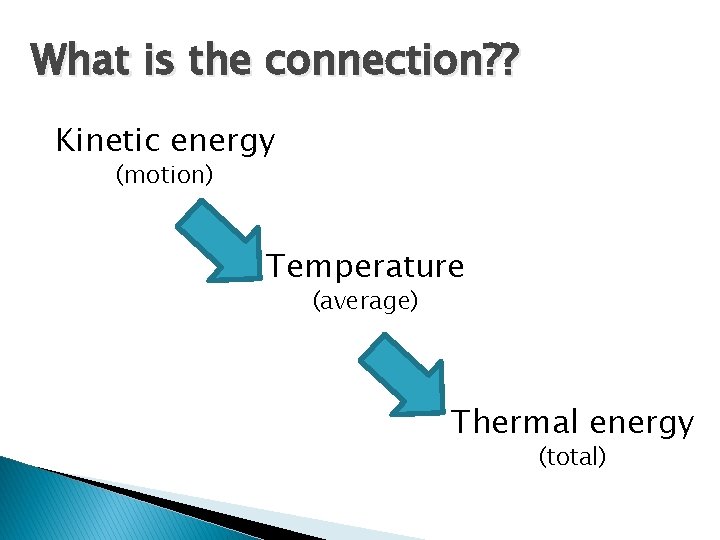
What is the connection? ? Kinetic energy (motion) Temperature (average) Thermal energy (total)
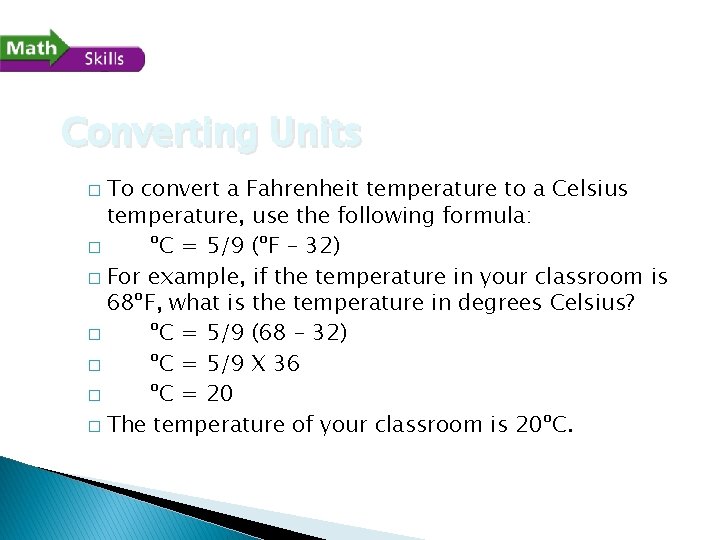
Converting Units To convert a Fahrenheit temperature to a Celsius temperature, use the following formula: � ºC = 5/9 (ºF – 32) � For example, if the temperature in your classroom is 68ºF, what is the temperature in degrees Celsius? � ºC = 5/9 (68 – 32) � ºC = 5/9 X 36 � ºC = 20 � The temperature of your classroom is 20ºC. �

Converting Units � � � Practice Problem While at the beach, you measure the ocean temperature as 77ºF. What is the temperature of the ocean in degrees Celsius? 25ºC

Bell Ringer �How do you use thermal energy in your everyday life?

Section 2: The Nature of Heat

- The Transfer of Heat Moves One Way � If two objects have different temperatures, heat will flow from the warmer object to the colder one.
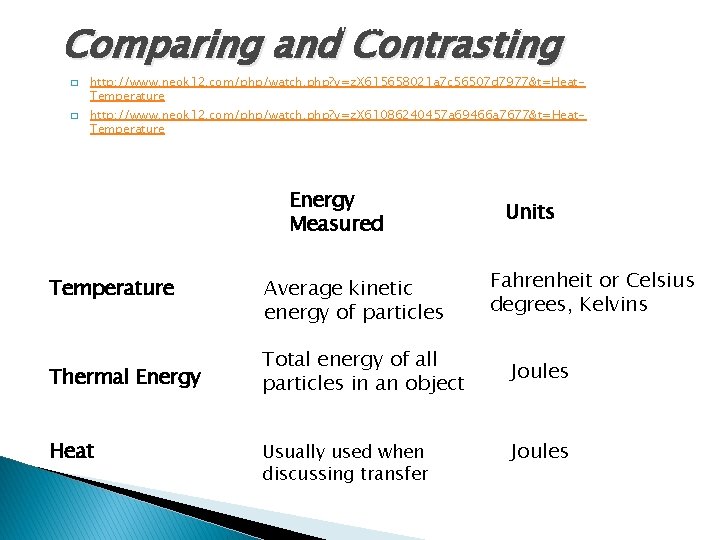
Temperature, Thermal Energy and Heat Comparing and Contrasting � � http: //www. neok 12. com/php/watch. php? v=z. X 615658021 a 7 c 56507 d 7977&t=Heat. Temperature http: //www. neok 12. com/php/watch. php? v=z. X 61086240457 a 69466 a 7677&t=Heat. Temperature Energy Measured Temperature Thermal Energy Heat Average kinetic energy of particles Total energy of all particles in an object Usually used when discussing transfer Units Fahrenheit or Celsius degrees, Kelvins Joules
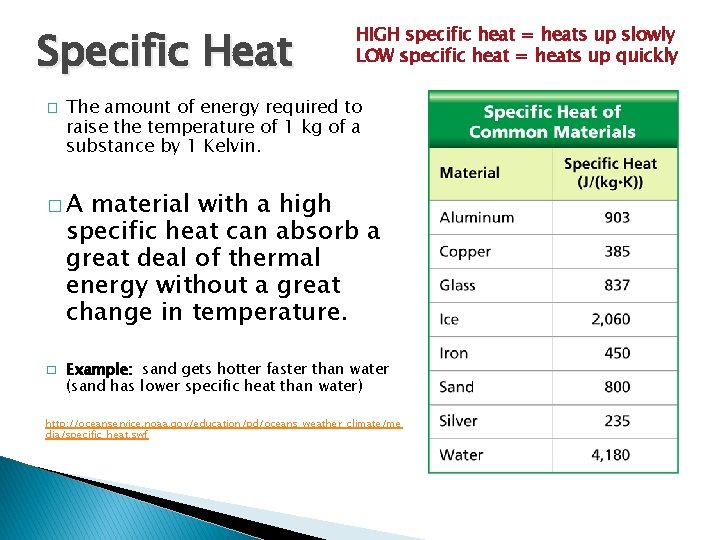
Specific Heat � Temperature, Thermal Heat HIGH Energy specificand heat = heats up slowly LOW specific heat = heats up quickly The amount of energy required to raise the temperature of 1 kg of a substance by 1 Kelvin. �A material with a high specific heat can absorb a great deal of thermal energy without a great change in temperature. � Example: sand gets hotter faster than water (sand has lower specific heat than water) http: //oceanservice. noaa. gov/education/pd/oceans_weather_climate/me dia/specific_heat. swf
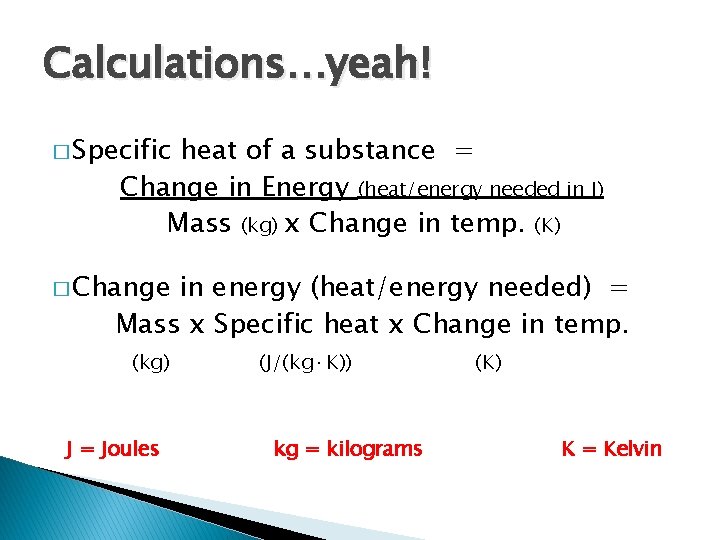
Calculations…yeah! � Specific heat of a substance = Change in Energy (heat/energy needed in J) Mass (kg) x Change in temp. (K) � Change in energy (heat/energy needed) = Mass x Specific heat x Change in temp. (kg) J = Joules (J/(kg·K)) kg = kilograms (K) K = Kelvin
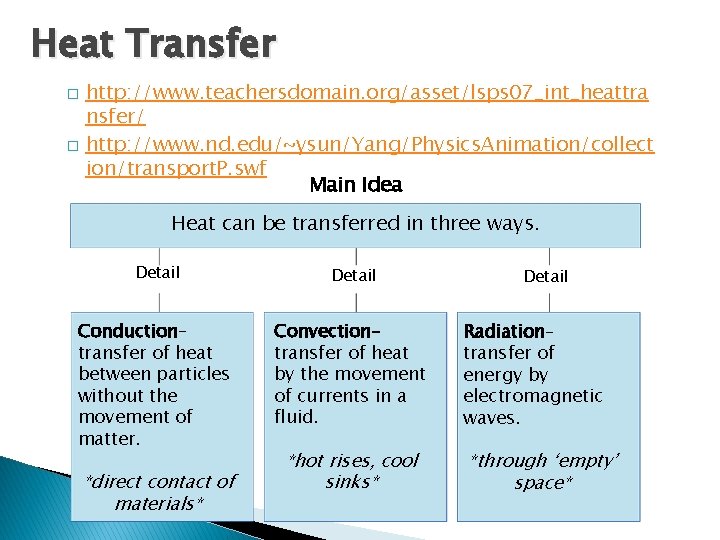
Heat Transfer � � - The Transfer of Heat http: //www. teachersdomain. org/asset/lsps 07_int_heattra nsfer/ http: //www. nd. edu/~ysun/Yang/Physics. Animation/collect ion/transport. P. swf Main Idea Heat can be transferred in three ways. Detail Conduction– transfer of heat between particles without the movement of matter. Convection– transfer of heat by the movement of currents in a fluid. *direct contact of materials* *hot rises, cool sinks* Detail Radiation– transfer of energy by electromagnetic waves. *through ‘empty’ space*

Bell Ringer �Do molecules move faster or slower when there is more thermal energy? Give me an example.

Section 3: Thermal Energy and States of Matter

Thermal Energy and States of Matter � Most matter on Earth can exist in three states –solid, liquid, and gas.
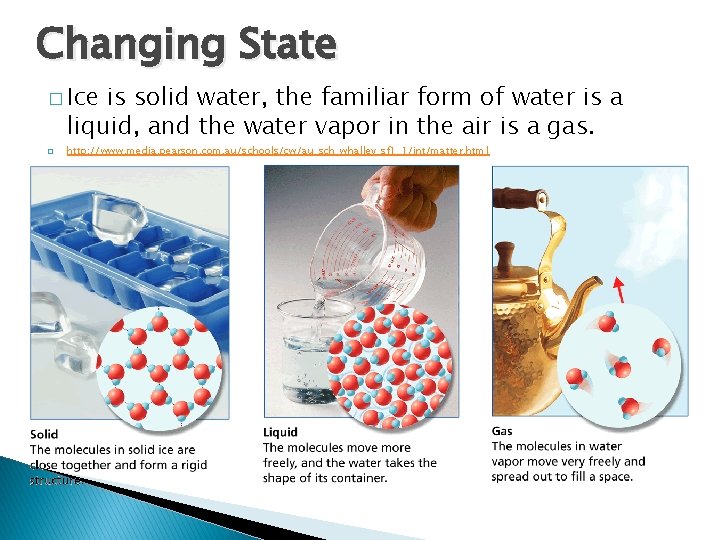
- The Properties of Water Changing State � Ice is solid water, the familiar form of water is a liquid, and the water vapor in the air is a gas. � http: //www. media. pearson. com. au/schools/cw/au_sch_whalley_sf 1_1/int/matter. html
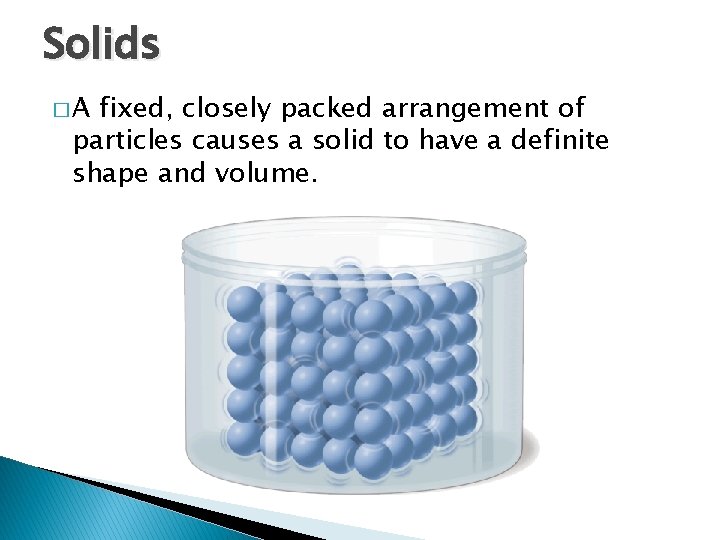
Solids �A - States of Matter fixed, closely packed arrangement of particles causes a solid to have a definite shape and volume.

Liquids � Because - States of Matter its particles are free to move, a liquid has no definite shape. However, it does have a definite volume.

Gases � As - States of Matter they move, gas particles spread apart, filling all the space available. Thus, a gas has neither definite shape nor definite volume.
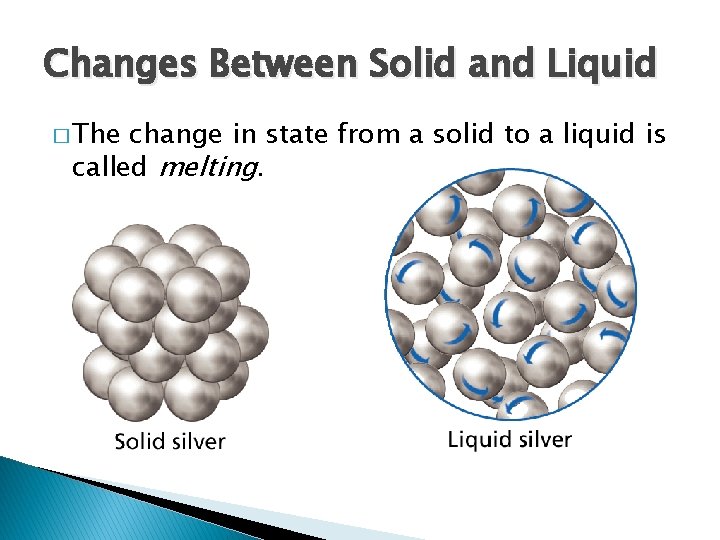
- Changes of State Changes Between Solid and Liquid � The change in state from a solid to a liquid is called melting.
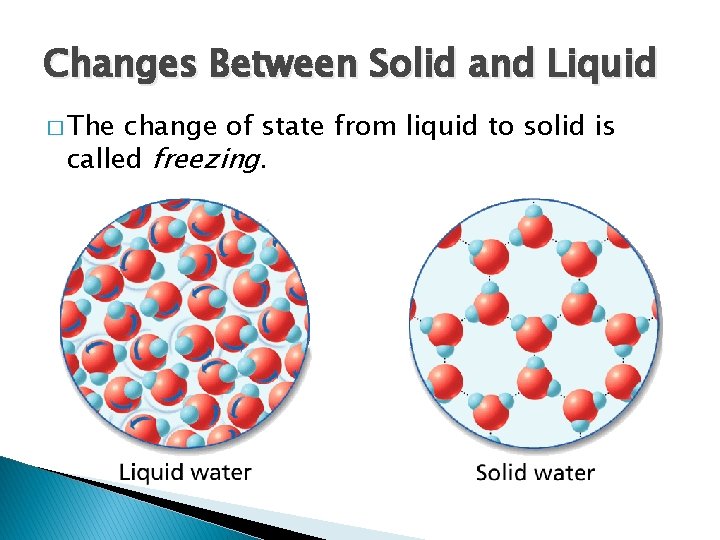
- Changes of State Changes Between Solid and Liquid � The change of state from liquid to solid is called freezing.
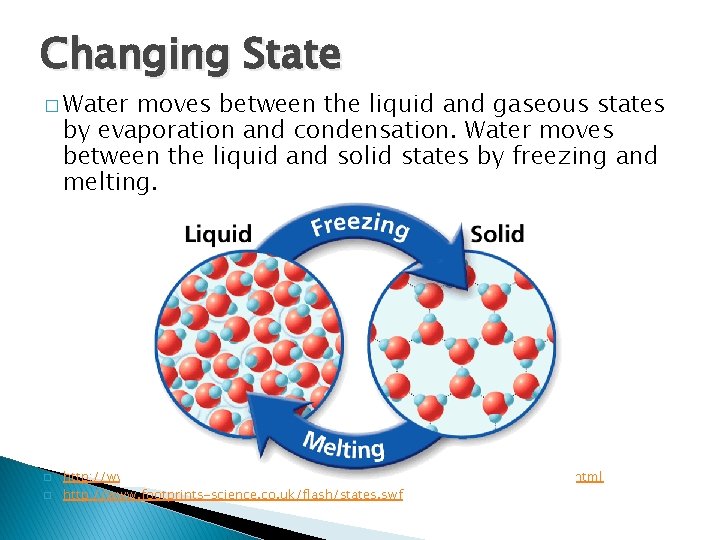
- The Properties of Water Changing State � Water moves between the liquid and gaseous states by evaporation and condensation. Water moves between the liquid and solid states by freezing and melting. � � http: //www. media. pearson. com. au/schools/cw/au_sch_whalley_sf 1_1/int/2_slg. html http: //www. footprints-science. co. uk/flash/states. swf
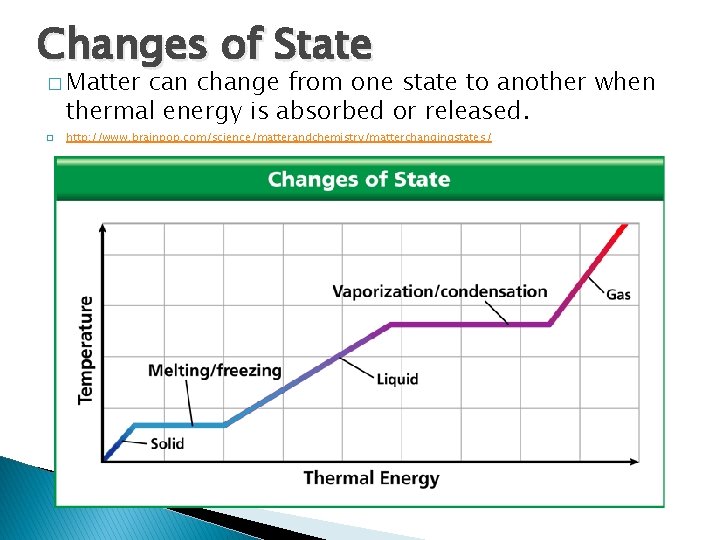
Thermal Energy and States of Matter Changes of State � Matter can change from one state to another when thermal energy is absorbed or released. � http: //www. brainpop. com/science/matterandchemistry/matterchangingstates/
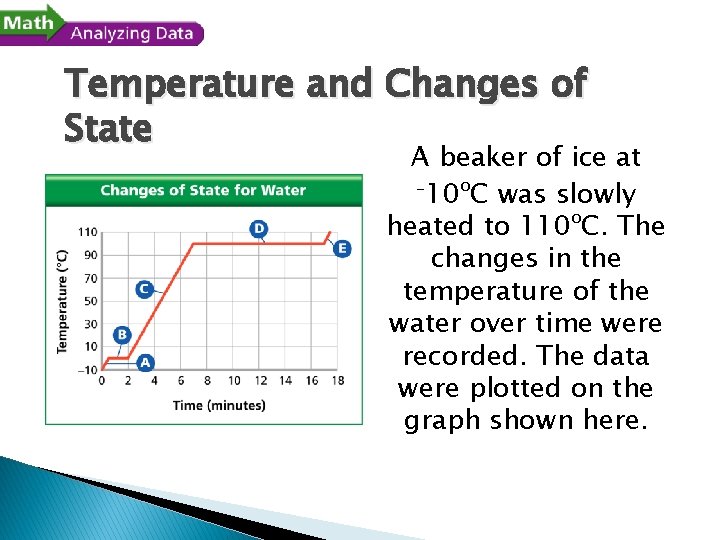
- Changes of State Temperature and Changes of State A beaker of ice at – 10ºC was slowly heated to 110ºC. The changes in the temperature of the water over time were recorded. The data were plotted on the graph shown here.
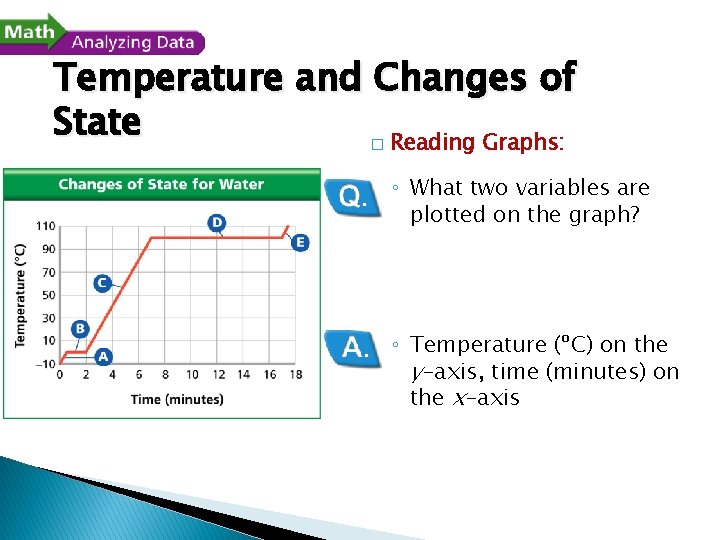
Temperature and Changes of State � Reading Graphs: ◦ What two variables are plotted on the graph? ◦ Temperature (ºC) on the y-axis, time (minutes) on the x-axis
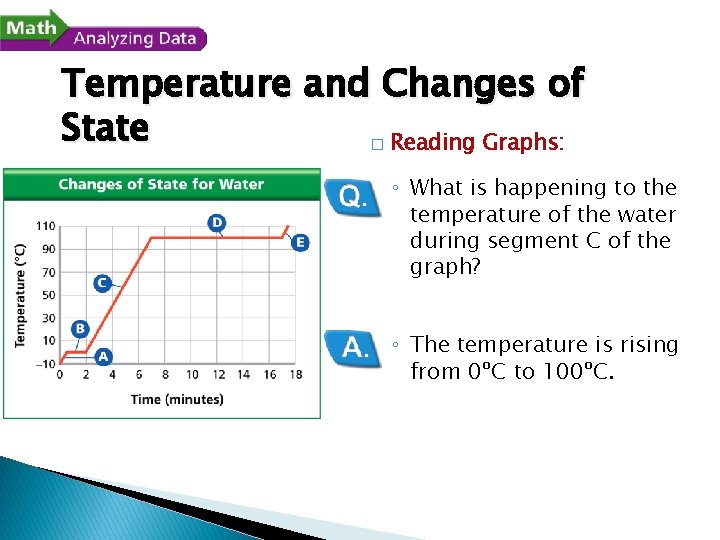
Temperature and Changes of State � Reading Graphs: ◦ What is happening to the temperature of the water during segment C of the graph? ◦ The temperature is rising from 0ºC to 100ºC.
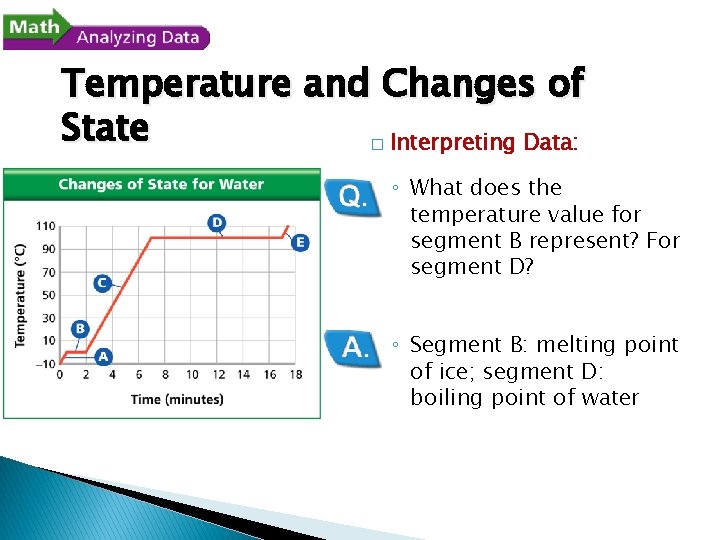
Temperature and Changes of State � Interpreting Data: ◦ What does the temperature value for segment B represent? For segment D? ◦ Segment B: melting point of ice; segment D: boiling point of water
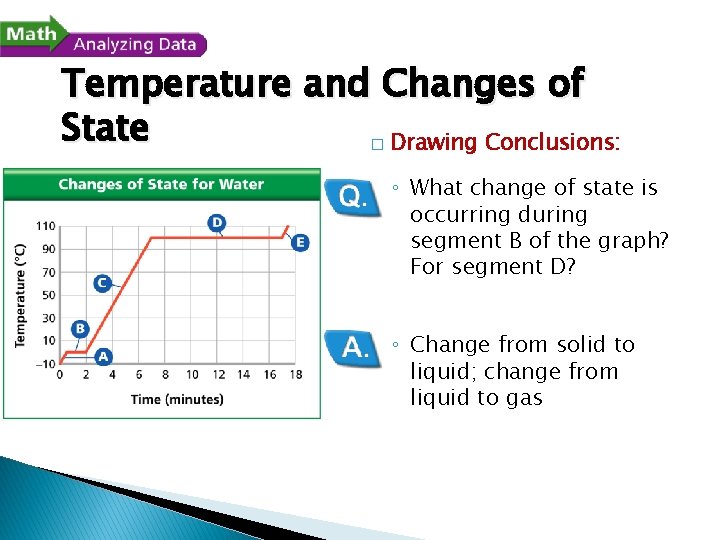
Temperature and Changes of State � Drawing Conclusions: ◦ What change of state is occurring during segment B of the graph? For segment D? ◦ Change from solid to liquid; change from liquid to gas
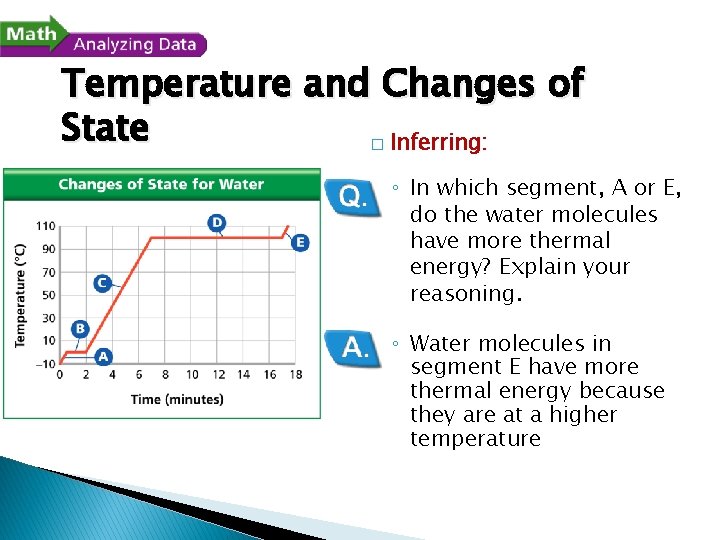
Temperature and Changes of State � Inferring: ◦ In which segment, A or E, do the water molecules have more thermal energy? Explain your reasoning. ◦ Water molecules in segment E have more thermal energy because they are at a higher temperature.
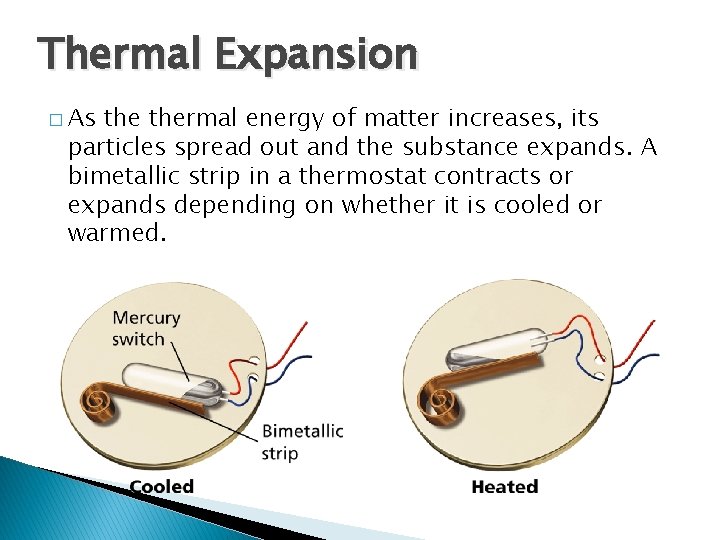
Thermal Energy and States of Matter Thermal Expansion � As thermal energy of matter increases, its particles spread out and the substance expands. A bimetallic strip in a thermostat contracts or expands depending on whether it is cooled or warmed.
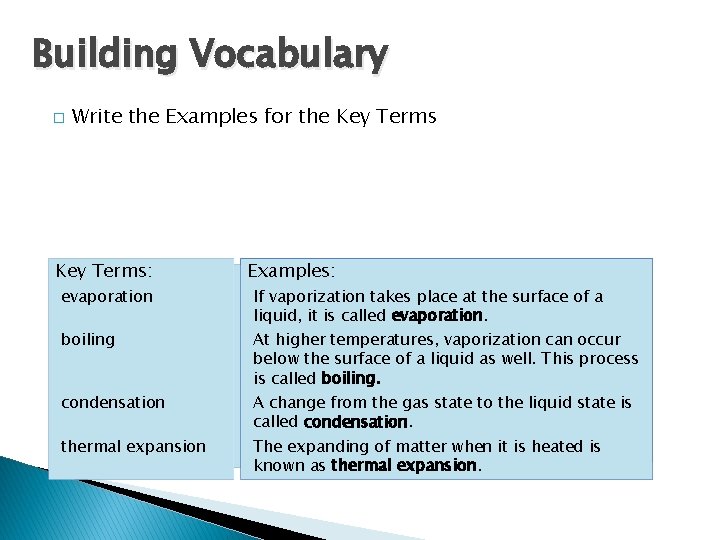
Thermal Energy and States of Matter Building Vocabulary � Write the Examples for the Key Terms Key Terms: evaporation state change of state boiling melting condensation freezing thermal expansion Examples: If vaporization Water can existtakes in three place different at the surface states, or of forms. a liquid, it is called evaporation. The physical change from one state of matter to At higheristemperatures, vaporization another called a change of state. can occur below the surface of a liquid as well. This process The change of state from a solid to a liquid is is called boiling. called melting. A change from the gas state to the liquid state is called condensation. The change of state from a liquid to a solid is The expanding called freezing. of matter when it is heated is known as thermal expansion.

Bell Ringer �Convert these following temperatures from degrees Fahrenheit to degrees Celcius: � 36 � 212 � 42

Electricity �How does Electricity impact your everyday life?
- Slides: 38