There are two categories of substances 1 Pure


There are two categories of substances. 1. Pure Substances AND 2. Mixtures

1. Pure Substances §A pure substance contains only one type of particle. §It is either an element or a compound.

ELEMENT §Is the simplest form of matter. §Has a unique set of properties. §Cannot chemically be broken down into simpler parts. §Contains only one type of atom. §They are all listed on the Periodic Table of Elements.
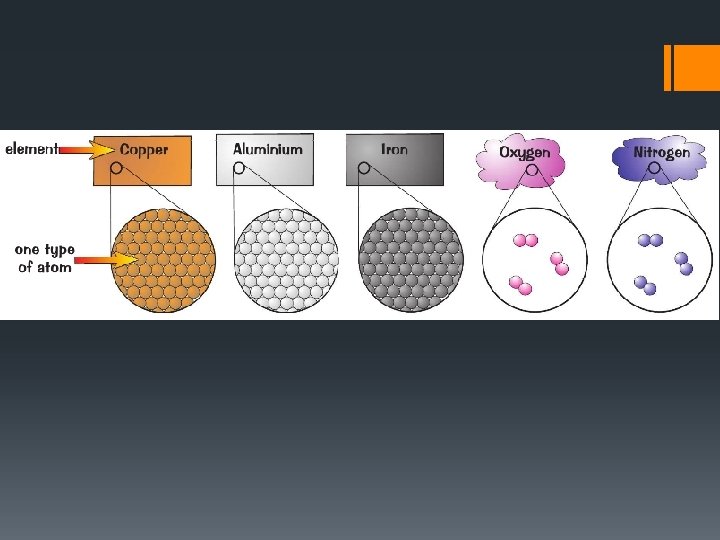

COMPOUND §A compound is formed when two or more different elements combine together. §When elements combine together they are called molecules. §Are able to be broken down into smaller parts called atoms.
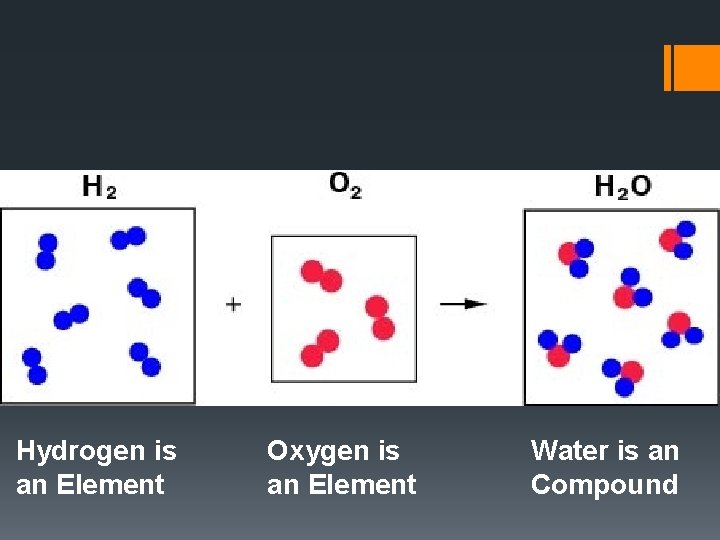
Hydrogen is an Element Oxygen is an Element Water is an Compound
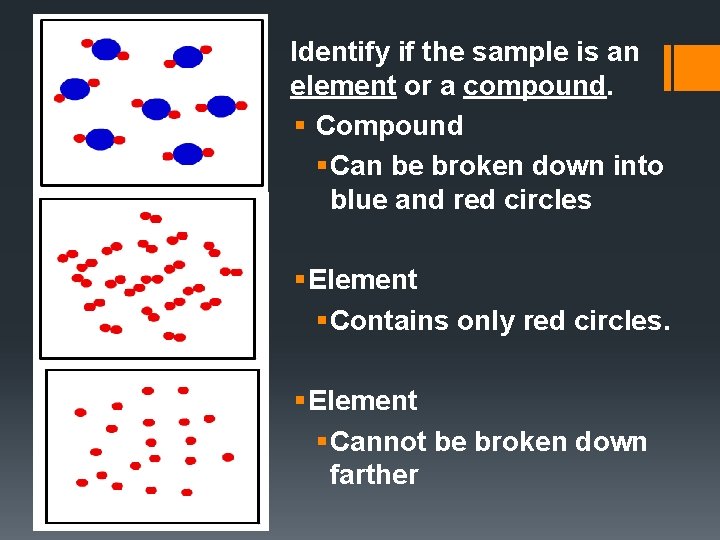
Identify if the sample is an element or a compound. § Compound § Can be broken down into blue and red circles § Element § Contains only red circles. § Element § Cannot be broken down farther

2. Mixture §A mixture contains at least two different pure substances, or two different types of particles. §The substances are not chemically combined. §They can be easily separated.

There are two types of mixtures Solutions AND Mechanical Mixtures

Solutions §A solution is a mixture that is made of two or more substances. §Only one phase is observed. §The components are evenly distributed and not easily distinguished. §Liquid and gas mixtures will be clear.


ALLOYS §An alloy is a special type of solution that is a mixture of two or more metals. §Bronze is an alloy of copper and tin


Mechanical Mixtures §A mechanical mixture is a mixture that is not uniform in composition. §Two or more phases are observed. (either with the eye or with a microscope) §Liquid and gas mechanical mixtures will be murky or opaque


Separating Matter §Mixtures can be separated in a number of ways. §Two of the most common separation techniques are filtration and distillation.
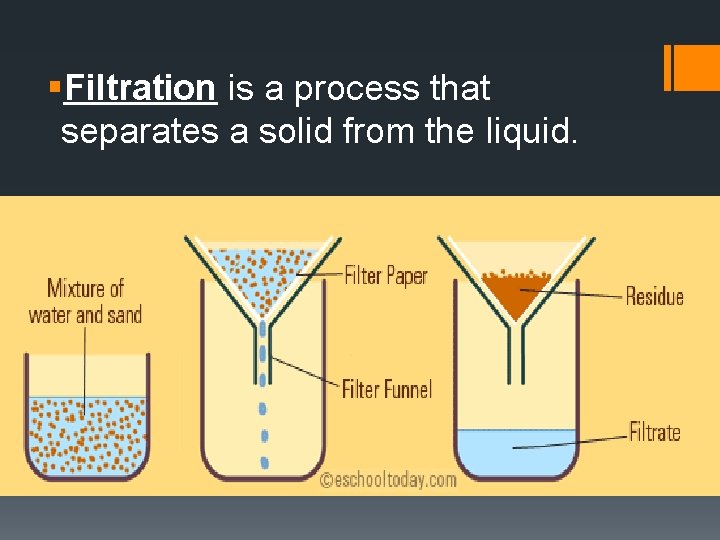
§Filtration is a process that separates a solid from the liquid.

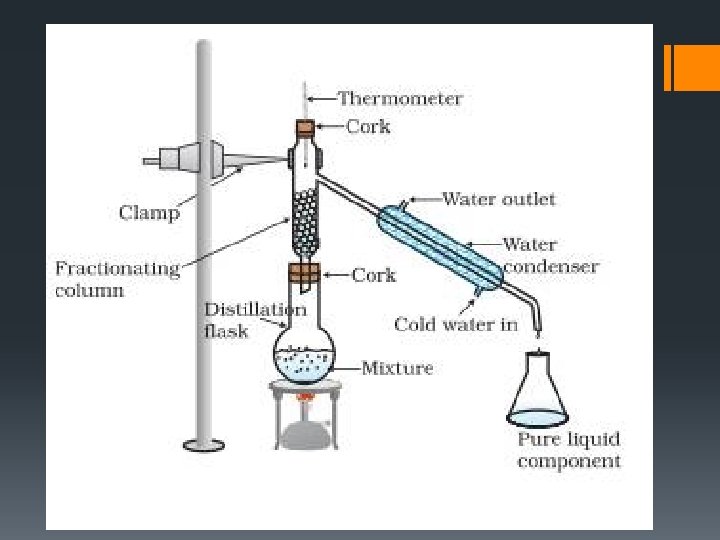
§Distillation is a process used to separate liquids based on differing boiling points. §The liquid with the lowest boiling point boils off first to produce a vapour that is then condensed back into a liquid and collected.


Practice §Handout §Text Page 178 #3 -8
- Slides: 22